The Value of Partial HPV Genotyping After Conization of Cervical Dysplasias
- PMID: 28845053
- PMCID: PMC5570588
- DOI: 10.1055/s-0043-115395
The Value of Partial HPV Genotyping After Conization of Cervical Dysplasias
Abstract
Introduction: In this retrospective study partial genotyping of human papilloma viruses (HPV) using the Abbott RealTime HighRisk HPV Test (RealTime) was compared with simple HPV detection (Qiagen Hybrid Capture 2 Test; hc2) for recurrence prediction at the first follow-up examination after conization of cervical intraepithelial neoplasia (CIN).
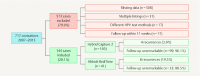
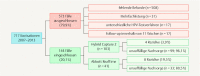
Methods: 144 women who had undergone conization for CIN between January 2007 and December 2013 were included. HPV status was determined preoperatively and at first follow-up using hc2 in 103 women and RealTime in 41 women. Recurrent or persistent CIN was assumed when CIN2+ was confirmed histologically or on comparable cytology findings.
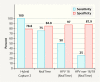
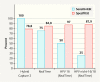
Results: Of the 144 women with complete data 12 (8.3%) had a recurrence after conization. HPV persistence at follow-up correlated significantly with recurrence (hc2: p = 0.003; RealTime: p = 0.003) and both sensitivity and specificity were high (hc2 = 100 and 78.4% respectively; RealTime = 75.0 and 83.9%). Whereas isolated HPV testing had a relatively low positive predictive value for recurrence (hc2 16%; RealTime 54.5%), this rose to 80% with HPV 16 detection at follow-up.
Conclusion: At follow-up after conization of CIN the combination of high risk HPV detection and partial genotyping of HPV 16 constitutes excellent diagnostic criteria for recurrence/persistence of CIN.
Einleitung In dieser retrospektiven Studie wurde die partielle Genotypisierung humaner Papillomviren (HPV) mithilfe des Abbott RealTime HighRisk HPV Tests (RealTime) verglichen mit dem einfachen HPV-Nachweis (Qiagen Hybrid Capture 2 Test; hc2) zur Vorhersage eines Rezidivs in der 1. Follow-up-Untersuchung nach Konisation bei zervikaler intraepithelialer Neoplasie (CIN). Methodik Es wurden 144 Frauen eingeschlossen, die im Zeitraum von Januar 2007 bis Dezember 2013 aufgrund einer CIN einer Konisation unterzogen wurden. Der HPV-Status wurde präoperativ, sowie zur 1. Follow-up-Untersuchung bei 103 Frauen mit hc2 und bei 41 Frauen mit RealTime ermittelt. Von einem Rezidiv bzw. einer persistierenden CIN wurde ausgegangen bei histologisch gesicherter CIN2+ oder vergleichbarem zytologischem Befund. Ergebnisse Von 144 Frauen mit vollständigen Daten hatten 12 (8,3%) ein Rezidiv nach Konisation. Eine HPV-Persistenz im Follow-up korrelierte signifikant mit einem Rezidiv (hc2: p = 0,003 bzw. RealTime: p = 0,003) bei hoher Sensitivität bzw. Spezifität (hc2 = 100 bzw. 78,4%; RealTime = 75,0 bzw. 83,9%). Während der positiv prädiktive Wert der alleinigen HPV-Testung für ein Rezidiv relativ niedrig lag (hc2 16%; RealTime 54,5%), stieg dieser bei HPV-16-Nachweis im Follow-up auf 80% an. Schlussfolgerung Im Follow-up nach Konisation einer CIN liefert eine Kombination aus Hochrisiko-HPV-Nachweis und partieller Genotypisierung von HPV 16 sehr gute Testgütekriterien für ein Rezidiv bzw. eine Persistenz der CIN.
Keywords: HPV-genotyping; cervical intraepithelial neoplasia; conization; human papilloma virus.
Conflict of interest statement
Figures
Similar articles
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
-
Evaluation of a multiplex real time PCR assay for the detection of human papillomavirus infections on self-collected cervicovaginal lavage samples.J Virol Methods. 2013 Oct;193(1):131-4. doi: 10.1016/j.jviromet.2013.05.009. Epub 2013 May 23. J Virol Methods. 2013. PMID: 23707925
-
The Abbott RealTime High Risk HPV test is a clinically validated human papillomavirus assay for triage in the referral population and use in primary cervical cancer screening in women 30 years and older: a review of validation studies.Acta Dermatovenerol Alp Pannonica Adriat. 2013;22(2):43-7. Acta Dermatovenerol Alp Pannonica Adriat. 2013. PMID: 23836358 Review.
-
HPV Tests Comparison in the Detection and Follow-Up after Surgical Treatment of CIN2+ Lesions.Diagnostics (Basel). 2022 Sep 29;12(10):2359. doi: 10.3390/diagnostics12102359. Diagnostics (Basel). 2022. PMID: 36292048 Free PMC article.
-
Detection of residual/recurrent cervical disease after successful LEEP conization: the possible role of mRNA-HPV test.Curr Pharm Des. 2013;19(8):1450-7. Curr Pharm Des. 2013. PMID: 23016778
Cited by
-
Association Between Positive Human Papillomavirus Status After Conization and Disease Recurrence in Patients with Cervical Intraepithelial Neoplasia Grade 3.J Obstet Gynaecol India. 2021 Feb;71(1):66-71. doi: 10.1007/s13224-020-01368-8. Epub 2020 Sep 10. J Obstet Gynaecol India. 2021. PMID: 33814801 Free PMC article.
-
Accuracy of GynTect® Methylation Markers to Detect Recurrent Disease in Patients Treated for CIN3: A Proof-of-Concept Case-Control Study.Cancers (Basel). 2024 Aug 30;16(17):3022. doi: 10.3390/cancers16173022. Cancers (Basel). 2024. PMID: 39272880 Free PMC article.
-
Cytology and High-Risk Human Papillomavirus Test for Cervical Cancer Screening Assessment.Diagnostics (Basel). 2022 Jul 19;12(7):1748. doi: 10.3390/diagnostics12071748. Diagnostics (Basel). 2022. PMID: 35885651 Free PMC article.
-
Evaluation of Integrated HPV DNA as Individualized Biomarkers for the Detection of Recurrent CIN2/3 during Post-Treatment Surveillance.Cancers (Basel). 2021 Jul 1;13(13):3309. doi: 10.3390/cancers13133309. Cancers (Basel). 2021. PMID: 34282754 Free PMC article.
-
Reporting and Assessing the Quality of Diagnostic Accuracy Studies for Cervical Cancer Screening and Management.J Low Genit Tract Dis. 2020 Apr;24(2):157-166. doi: 10.1097/LGT.0000000000000527. J Low Genit Tract Dis. 2020. PMID: 32243311 Free PMC article.
References
-
- Bosch F X, Burchell A N, Schiffman M. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26 10:K1–K16. - PubMed
-
- Bouvard V, Baan R, Straif K. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10:321–322. - PubMed
-
- de Sanjose S, Quint W G, Alemany L. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. - PubMed
-
- Walboomers J M, Jacobs M V, Manos M M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
-
- Kalliala I, Nieminen P, Dyba T. Cancer free survival after CIN treatment: comparisons of treatment methods and histology. Gynecol Oncol. 2007;105:228–233. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

