Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells
- PMID: 28845214
- PMCID: PMC5563420
- DOI: 10.1155/2017/7054272
Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells
Abstract
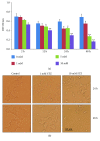
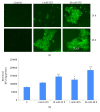
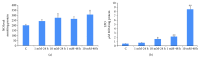
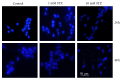
Streptozotocin is a pancreatic beta-cell-specific cytotoxin and is widely used to induce experimental type 1 diabetes in rodent models. The precise molecular mechanism of STZ cytotoxicity is however not clear. Studies have suggested that STZ is preferably absorbed by insulin-secreting β-cells and induces cytotoxicity by producing reactive oxygen species/reactive nitrogen species (ROS/RNS). In the present study, we have investigated the mechanism of cytotoxicity of STZ in insulin-secreting pancreatic cancer cells (Rin-5F) at different doses and time intervals. Cell viability, apoptosis, oxidative stress, and mitochondrial bioenergetics were studied. Our results showed that STZ induces alterations in glutathione homeostasis and inhibited the activities of the respiratory enzymes, resulting in inhibition of ATP synthesis. Apoptosis was observed in a dose- and time-dependent manner. Western blot analysis has also confirmed altered expression of oxidative stress markers (e.g., NOS and Nrf2), cell signaling kinases, apoptotic protein-like caspase-3, PARP, and mitochondrial specific proteins. These results suggest that STZ-induced cytotoxicity in pancreatic cells is mediated by an increase in oxidative stress, alterations in cellular metabolism, and mitochondrial dysfunction. This study may be significant in better understanding the mechanism of STZ-induced β-cell toxicity/resistance and the etiology of type 1 diabetes induction.
Figures
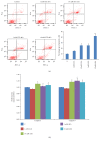
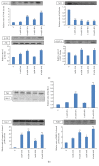
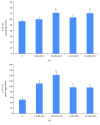
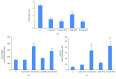

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

