Enriched housing promotes post-stroke functional recovery through astrocytic HMGB1-IL-6-mediated angiogenesis
- PMID: 28845299
- PMCID: PMC5563836
- DOI: 10.1038/cddiscovery.2017.54
Enriched housing promotes post-stroke functional recovery through astrocytic HMGB1-IL-6-mediated angiogenesis
Abstract
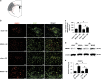
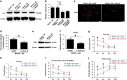
Enriched environment (EE) is shown to promote angiogenesis, neurogenesis and functional recovery after ischemic stroke. However, the underlying mechanisms remain unclear. C57BL/6 mice underwent middle cerebral artery occlusion (60 min) followed by reperfusion, after which mice were housed in either standard environment (SE) or EE. Here we found that post-ischemic EE exhibited decreased depression and anxiety-like behavior, and promoted angiogenesis and functional recovery compared to SE mice. EE mice treated with high-mobility group box-1 (HMGB1) inhibitor glycyrrhizin had an increased post-stroke depression and anxiety-like behavior, and the angiogenesis and functional recovery were decreased. HMGB1 and interleukin-6 (IL-6) expression in astrocyte were increased in EE mice. EE mice treated with glycyrrhizin decreased, whereas EE mice treated with recombinant HMGB1 (rHMGB1) increased the levels of IL-6 and p-AKT. Blockade of IL-6 with anti-IL-6-neutralizing antibody in EE mice attenuated EE-mediated angiogenesis and functional recovery. Furthermore, our in vitro data revealed that in primary astrocyte cultures rHMGB1 promoted the expression of IL-6 in activated astrocytes. PI3K/AKT signaling pathway was involved in HMGB1-mediated expression of astrocytic IL-6. Thus, our results reveal a previously uncharacterized property of HMGB1/IL-6 signaling pathway in EE-mediated angiogenesis and functional recovery after ischemic stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 2008; 371: 1612–1623. - PubMed
-
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002). Prog Neurobiol 2004; 72: 167–182. - PubMed
-
- Keyvani K, Sachser N, Witte OW, Paulus W. Gene expression profiling in the intact and injured brain following environmental enrichment. J Neuropathol Exp Neurol 2004; 63: 598–609. - PubMed
-
- Johansson BB. Functional and cellular effects of environmental enrichment after experimental brain infarcts. Restor Neurol Neurosci 2004; 22: 163–174. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

