The Safety and Immunogenicity of Live Zoster Vaccination in Patients With Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial
- PMID: 28845577
- PMCID: PMC5656925
- DOI: 10.1002/art.40187
The Safety and Immunogenicity of Live Zoster Vaccination in Patients With Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial
Abstract
Objective: Patients with rheumatoid arthritis (RA) are at increased risk of herpes zoster, and vaccination is recommended for patients ages 50 years and older, prior to starting treatment with biologic agents or tofacitinib. Tofacitinib is an oral JAK inhibitor for the treatment of RA. We evaluated its effect on the immune response and safety of live zoster vaccine (LZV).
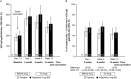
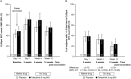
Methods: In this phase II, 14-week, placebo-controlled trial, patients ages 50 years and older who had active RA and were receiving background methotrexate were given LZV and randomized to receive tofacitinib 5 mg twice daily or placebo 2-3 weeks postvaccination. We measured humoral responses (varicella zoster virus [VZV]-specific IgG level as determined by glycoprotein enzyme-linked immunosorbent assay) and cell-mediated responses (VZV-specific T cell enumeration, as determined by enzyme-linked immunospot assay) at baseline and 2 weeks, 6 weeks, and 14 weeks postvaccination. End points included the geometric mean fold rise (GMFR) in VZV-specific IgG levels (primary end point) and T cells (number of spot-forming cells/106 peripheral blood mononuclear cells) at 6 weeks postvaccination.
Results: One hundred twelve patients were randomized to receive tofacitinib (n = 55) or placebo (n = 57). Six weeks postvaccination, the GMFR in VZV-specific IgG levels was 2.11 in the tofacitinib group and 1.74 in the placebo group, and the VZV-specific T cell GMFR was similar in the tofacitinib group and the placebo group (1.50 and 1.29, respectively). Serious adverse events occurred in 3 patients in the tofacitinib group (5.5%) and 0 patients (0.0%) in the placebo group. One patient, who lacked preexisting VZV immunity, developed cutaneous vaccine dissemination 2 days after starting tofacitinib (16 days postvaccination). This resolved after tofacitinib was discontinued and the patient received antiviral treatment.
Conclusion: Patients who began treatment with tofacitinib 2-3 weeks after receiving LZV had VZV-specific humoral and cell-mediated immune responses to LZV similar to those in placebo-treated patients. Vaccination appeared to be safe in all of the patients except 1 patient who lacked preexisting VZV immunity.
Trial registration: ClinicalTrials.gov NCT02147587.
© 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures
Comment in
-
Editorial: Herpes Zoster: Fear the Infection, Value the Solution.Arthritis Rheumatol. 2017 Oct;69(10):1917-1920. doi: 10.1002/art.40188. Arthritis Rheumatol. 2017. PMID: 28845573 No abstract available.
-
Rheumatoid arthritis: Reducing the risk of herpes zoster.Nat Rev Rheumatol. 2017 Nov;13(11):634. doi: 10.1038/nrrheum.2017.153. Epub 2017 Sep 14. Nat Rev Rheumatol. 2017. PMID: 28905860 No abstract available.
References
-
- Harpaz R, Ortega‐Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57(RR‐5):1–30. - PubMed
-
- Smolen J, Genovese M, Takeuchi T, Hyslop D, Macias WL, Rooney TP, et al. Safety profile of baricitinib in patients with active RA: integrated analysis [abstract]. Ann Rheum Dis 2016;75:THU0166.
-
- Jung CW, Shih LY, Xiao Z, Jie J, Hou HA, Du X, et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk Lymphoma 2015;56:2067–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

