Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy
- PMID: 28845604
- PMCID: PMC5656820
- DOI: 10.1002/art.40189
Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy
Abstract
Objective: Patients with rheumatoid arthritis (RA) are at increased risk of herpes zoster (HZ), and the risk appears to be increased in patients treated with tofacitinib. The aim of this study was to evaluate whether concomitant treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or glucocorticoids (GCs) contributes to the increased risk of HZ in RA patients treated with tofacitinib.
Methods: HZ cases were identified from the databases of 2 phase I, 9 phase II, 6 phase III, and 2 long-term extension studies of tofacitinib in RA patients. Crude incidence rates (IRs) of all HZ events (serious and nonserious) per 100 patient-years (with 95% confidence intervals [95% CIs]) were calculated for unique patients. Within phase III studies, we described HZ rates according to concomitant csDMARD treatment and baseline GC use. A multivariable Cox proportional hazards regression model was used to evaluate HZ risk factors across studies.
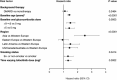
Results: Across all studies (6,192 patients; 16,839 patient-years), HZ was reported in 636 tofacitinib-treated patients (IR 4.0, 95% CI 3.7-4.4). In most cases (93%), HZ was classified as nonserious, and the majority of patients (94%) had involvement of only 1 dermatome. HZ IRs varied across regions, from 2.4 (95% CI 2.0-2.9) in Eastern Europe to 8.0 (95% CI 6.6-9.6) in Japan and 8.4 (95% CI 6.4-10.9) in Korea. Within phase III studies, HZ IRs varied according to tofacitinib dose, background csDMARD treatment, and baseline use of GCs. The IRs were numerically lowest for monotherapy with tofacitinib 5 mg twice daily without GCs (IR 0.56 [95% CI 0.07-2.01]) and highest for tofacitinib 10 mg twice daily with csDMARDs and GCs (IR 5.44 [95% CI 3.72-7.68]). Age, GC use, tofacitinib dose, and enrollment within Asia were independent risk factors for HZ.
Conclusion: Patients receiving treatment with tofacitinib and GCs appear to have a greater risk of developing HZ compared with patients receiving tofacitinib monotherapy without GCs.
© 2017 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

Comment in
-
Rheumatoid arthritis: Reducing the risk of herpes zoster.Nat Rev Rheumatol. 2017 Nov;13(11):634. doi: 10.1038/nrrheum.2017.153. Epub 2017 Sep 14. Nat Rev Rheumatol. 2017. PMID: 28905860 No abstract available.
References
-
- Harpaz R, Ortega‐Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57(RR–5):1–30. - PubMed
-
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002;18:350–4. - PubMed
-
- Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007;57:1431–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

