β-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice
- PMID: 28846687
- PMCID: PMC5591015
- DOI: 10.1371/journal.pgen.1006990
β-catenin is required for taste bud cell renewal and behavioral taste perception in adult mice
Abstract
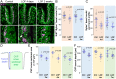
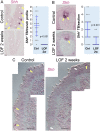
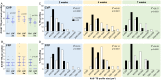
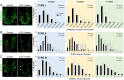
Taste stimuli are transduced by taste buds and transmitted to the brain via afferent gustatory fibers. Renewal of taste receptor cells from actively dividing progenitors is finely tuned to maintain taste sensitivity throughout life. We show that conditional β-catenin deletion in mouse taste progenitors leads to rapid depletion of progenitors and Shh+ precursors, which in turn causes taste bud loss, followed by loss of gustatory nerve fibers. In addition, our data suggest LEF1, TCF7 and Wnt3 are involved in a Wnt pathway regulatory feedback loop that controls taste cell renewal in the circumvallate papilla epithelium. Unexpectedly, taste bud decline is greater in the anterior tongue and palate than in the posterior tongue. Mutant mice with this regional pattern of taste bud loss were unable to discern sweet at any concentration, but could distinguish bitter stimuli, albeit with reduced sensitivity. Our findings are consistent with published reports wherein anterior taste buds have higher sweet sensitivity while posterior taste buds are better tuned to bitter, and suggest β-catenin plays a greater role in renewal of anterior versus posterior taste buds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates.PLoS Genet. 2015 May 28;11(5):e1005208. doi: 10.1371/journal.pgen.1005208. eCollection 2015 May. PLoS Genet. 2015. PMID: 26020789 Free PMC article.
-
Maintenance of Taste Organs Is Strictly Dependent on Epithelial Hedgehog/GLI Signaling.PLoS Genet. 2016 Nov 28;12(11):e1006442. doi: 10.1371/journal.pgen.1006442. eCollection 2016 Nov. PLoS Genet. 2016. PMID: 27893742 Free PMC article.
-
β-Catenin signaling regulates temporally discrete phases of anterior taste bud development.Development. 2015 Dec 15;142(24):4309-17. doi: 10.1242/dev.121012. Epub 2015 Nov 2. Development. 2015. PMID: 26525674 Free PMC article.
-
Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation.Int J Mol Sci. 2019 Mar 16;20(6):1341. doi: 10.3390/ijms20061341. Int J Mol Sci. 2019. PMID: 30884865 Free PMC article. Review.
-
How taste works: cells, receptors and gustatory perception.Cell Mol Biol Lett. 2015 Dec;20(5):699-716. doi: 10.1515/cmble-2015-0042. Cell Mol Biol Lett. 2015. PMID: 26447485 Review.
Cited by
-
Organoid in dentistry: Models for oral biology and disease.J Dent Sci. 2025 Jul;20(3):1816-1823. doi: 10.1016/j.jds.2025.05.002. Epub 2025 May 15. J Dent Sci. 2025. PMID: 40654442 Free PMC article. Review.
-
Changes in the rumen development, rumen fermentation, and rumen microbiota community in weaned calves during steviol glycosides treatment.Front Microbiol. 2024 Jun 24;15:1395665. doi: 10.3389/fmicb.2024.1395665. eCollection 2024. Front Microbiol. 2024. PMID: 38979539 Free PMC article.
-
Targeting ligand-dependent wnt pathway dysregulation in gastrointestinal cancers through porcupine inhibition.Pharmacol Ther. 2022 Oct;238:108179. doi: 10.1016/j.pharmthera.2022.108179. Epub 2022 Mar 28. Pharmacol Ther. 2022. PMID: 35358569 Free PMC article. Review.
-
Anterior and Posterior Tongue Regions and Taste Papillae: Distinct Roles and Regulatory Mechanisms with an Emphasis on Hedgehog Signaling and Antagonism.Int J Mol Sci. 2023 Mar 2;24(5):4833. doi: 10.3390/ijms24054833. Int J Mol Sci. 2023. PMID: 36902260 Free PMC article. Review.
-
SOX2 regulates homeostasis of taste bud cells and lingual epithelial cells in posterior tongue.PLoS One. 2020 Oct 15;15(10):e0240848. doi: 10.1371/journal.pone.0240848. eCollection 2020. PLoS One. 2020. PMID: 33057384 Free PMC article.
References
-
- Ninomiya Y, Kajiura H, Mochizuki K. Differential taste responses of mouse chorda tympani and glossopharyngeal nerves to sugars and amino acids. Neuroscience letters. 1993;163(2):197–200. . - PubMed
-
- Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comparative Biochemistry and Physiology Part A: Physiology. 1989;92(3):371–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

