Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis
- PMID: 28847008
- PMCID: PMC5573680
- DOI: 10.1371/journal.pone.0183506
Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis
Abstract
Aim: To evaluate the effects of metformin (Met) on inflammation, oxidative stress, and bone loss in a rat model of ligature-induced periodontitis.
Materials & methods: Male albino Wistar rats were divided randomly into five groups of twenty-one rats each, and given the following treatments for 10 days: (1) no ligature + water, (2) ligature + water, (3) ligature + 50 mg/kg Met, (4) ligature + 100 mg/kg Met, and (5) ligature + 200 mg/kg Met. Water or Met was administered orally. Maxillae were fixed and scanned using Micro-computed Tomography (μCT) to quantitate linear and bone volume/tissue volume (BV/TV) volumetric bone loss. Histopathological characteristics were assessed through immunohistochemical staining for MMP-9, COX-2, the RANKL/RANK/OPG pathway, SOD-1, and GPx-1. Additionally, confocal microscopy was used to analyze osteocalcin fluorescence. UV-VIS analysis was used to examine the levels of malondialdehyde, glutathione, IL-1β and TNF-α from gingival tissues. Quantitative RT-PCR reaction was used to gene expression of AMPK, NF-κB (p65), and Hmgb1 from gingival tissues. Significance among groups were analysed using a one-way ANOVA. A p-value of p<0.05 indicated a significant difference.
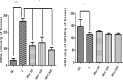
Results: Treatment with 50 mg/kg Met significantly reduced concentrations of malondialdehyde, IL-1β, and TNF-α (p < 0.05). Additionally, weak staining was observed for COX-2, MMP-9, RANK, RANKL, SOD-1, and GPx-1 after 50 mg/kg Met. OPG and Osteocalcin showed strong staining in the same group. Radiographically, linear measurements showed a statistically significant reduction in bone loss after 50 mg/kg Met compared to the ligature and Met 200 mg/kg groups. The same pattern was observed volumetrically in BV/TV and decreased osteoclast number (p<0.05). RT-PCR showed increased AMPK expression and decreased expression of NF-κB (p65) and HMGB1 after 50 mg/kg Met.
Conclusions: Metformin, at a concentration of 50 mg/kg, decreases the inflammatory response, oxidative stress and bone loss in ligature-induced periodontitis in rats.
Conflict of interest statement
Figures








References
-
- Henderson B, Nair SP, Ward JM, Wilson M (2003) Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol 57: 29–55. doi: 10.1146/annurev.micro.57.030502.090908 - DOI - PubMed
-
- Al-Khabbaz AK (2014) Type 2 diabetes mellitus and periodontal disease severity. Oral Health Prev Dent 12: 77–82. doi: 10.3290/j.ohpd.a31223 - DOI - PubMed
-
- Demmer RT, Holtfreter B, Desvarieux M, Jacobs DR Jr., Kerner W, Nauck M, et al. (2012) The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 35: 2036–2042. doi: 10.2337/dc11-2453 - DOI - PMC - PubMed
-
- Woo V (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes: response to Nathan et al. Diabetes Care 32: e34; author reply e37-38. doi: 10.2337/dc08-2093 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

