Bacterial Heme-Based Sensors of Nitric Oxide
- PMID: 28847157
- PMCID: PMC6217740
- DOI: 10.1089/ars.2017.7235
Bacterial Heme-Based Sensors of Nitric Oxide
Abstract
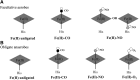
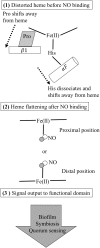
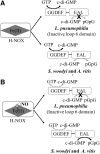
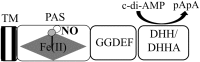
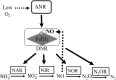
Significance: The molecule nitric oxide (NO) has been shown to regulate behaviors in bacteria, including biofilm formation. NO detection and signaling in bacteria is typically mediated by hemoproteins such as the bis-(3',5')-cyclic dimeric adenosine monophosphate-specific phosphodiesterase YybT, the transcriptional regulator dissimilative nitrate respiration regulator, or heme-NO/oxygen binding (H-NOX) domains. H-NOX domains are well-characterized primary NO sensors that are capable of detecting nanomolar NO and influencing downstream signal transduction in many bacterial species. However, many bacteria, including the human pathogen Pseudomonas aeruginosa, respond to nanomolar concentrations of NO but do not contain an annotated H-NOX domain, indicating the existence of an additional nanomolar NO-sensing protein (NosP). Recent Advances: A newly discovered bacterial hemoprotein called NosP may also act as a primary NO sensor in bacteria, in addition to, or in place of, H-NOX. NosP was first described as a regulator of a histidine kinase signal transduction pathway that is involved in biofilm formation in P. aeruginosa.
Critical issues: The molecular details of NO signaling in bacteria are still poorly understood. There are still many bacteria that are NO responsive but do encode either H-NOX or NosP domains in their genomes. Even among bacteria that encode H-NOX or NosP, many questions remain.
Future directions: The molecular mechanisms of NO regulation in many bacteria remain to be established. Future studies are required to gain knowledge about the mechanism of NosP signaling. Advancements on structural and molecular understanding of heme-based sensors in bacteria could lead to strategies to alleviate or control bacterial biofilm formation or persistent biofilm-related infections.
Keywords: H-NOX; NO sensor; NosP; hemoprotein; nitric oxide; signal transduction.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Arora DP. and Boon EM. Nitric oxide regulated two-component signaling in Pseudoalteromonas atlantica. Biochem Biophys Res Commun 421: 521–526, 2012 - PubMed
-
- Arora DP, Hossain S, Xu Y, and Boon EM. Nitric oxide regulation of bacterial biofilms. Biochemistry 54: 3717–3728, 2015 - PubMed
-
- Barraud N, Kelso MJ, Rice SA, and Kjelleberg S. Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des 21: 31–42, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

