Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study
- PMID: 28847636
- PMCID: PMC5705273
- DOI: 10.1016/S1473-3099(17)30488-7
Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study
Abstract
Background: Symptom-based screening for tuberculosis is recommended for all people living with HIV. This recommendation results in unnecessary Xpert MTB/RIF testing in many individuals living in tuberculosis-endemic areas and thus poor implementation of intensified case finding and tuberculosis preventive therapy. Novel approaches to tuberculosis screening are needed to help achieve global targets for tuberculosis elimination. We assessed the performance of C-reactive protein (CRP) measured with a point-of-care assay as a screening tool for active pulmonary tuberculosis.
Methods: For this prospective study, we enrolled adults (aged ≥18 years) living with HIV with CD4 cell count less than or equal to 350 cells per μL who were initiating antiretroviral therapy (ART) from two HIV/AIDS clinics in Uganda. CRP concentrations were measured at study entry with a point-of-care assay using whole blood obtained by fingerprick (concentration ≥10 mg/L defined as screen positive for tuberculosis). Sputum samples were collected for Xpert MTB/RIF testing and culture. We calculated the sensitivity and specificity of point-of-care CRP and WHO symptom-based screening in reference to culture results. We repeated the sensitivity analysis with Xpert MTB/RIF as the reference standard.
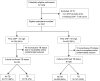
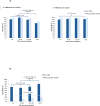
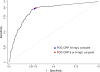
Findings: Between July 8, 2013, and Dec 15, 2015, 1237 HIV-infected adults were enrolled and underwent point-of-care CRP testing. 60 (5%) patients with incomplete or contaminated cultures were excluded from the analysis. Of the remaining 1177 patients (median CD4 count 165 cells per μL [IQR 75-271]), 163 (14%) had culture-confirmed tuberculosis. Point-of-care CRP testing had 89% sensitivity (145 of 163, 95% CI 83-93) and 72% specificity (731 of 1014, 95% CI 69-75) for culture-confirmed tuberculosis. Compared with WHO symptom-based screening, point-of-care CRP testing had lower sensitivity (difference -7%, 95% CI -12 to -2; p=0·002) but substantially higher specificity (difference 58%, 95% CI 55 to 61; p<0·0001). When Xpert MTB/RIF results were used as the reference standard, sensitivity of point-of-care CRP and WHO symptom-based screening were similar (94% [79 of 84] vs 99% [83 of 84], respectively; difference -5%, 95% CI -12 to 2; p=0·10).
Interpretation: The performance characteristics of CRP support its use as a tuberculosis screening test for people living with HIV with CD4 count less than or equal to 350 cells per μL who are initiating ART. HIV/AIDS programmes should consider point-of-care CRP-based tuberculosis screening to improve the efficiency of intensified case finding and increase uptake of tuberculosis preventive therapy.
Funding: National Institutes of Health; President's Emergency Plan for AIDS Relief; University of California, San Francisco, Nina Ireland Program for Lung Health.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
CRP: tell-tale biomarker or common denominator?Lancet Infect Dis. 2017 Dec;17(12):1225-1227. doi: 10.1016/S1473-3099(17)30472-3. Epub 2017 Aug 25. Lancet Infect Dis. 2017. PMID: 28847637 No abstract available.
References
-
- World Health Organization. Geneva, Switzerland: WHO; 2016. Global Tuberculosis Report. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?....
-
- World Health Organization. WHO; [Geneva, Switzerland]. 2013. Systematic screening for active tuberculosis: principles and recommendations. Available from: http://www.who.int/tb/tbscreening/en/ - PubMed
-
- World Health Organization. Geneva, Switzerland: WHO; 2014. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Available from: http://www.who.int/tb/publications/tpp_report/en/
-
- World Health Organization. Geneva, Switzerland: WHO; 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Available from: http://apps.who.int/iris/bitstream/10665/44472/1/9789241500708_eng.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

