Long-term results of endovascular repair for descending thoracic aortic aneurysms
- PMID: 28847657
- PMCID: PMC9799071
- DOI: 10.1016/j.jvs.2017.06.094
Long-term results of endovascular repair for descending thoracic aortic aneurysms
Abstract
Objective: Since thoracic endovascular aortic repair (TEVAR) received U.S. Food and Drug Administration approval for the treatment of descending thoracic aneurysms in March 2005, excellent 30-day and midterm outcomes have been described. However, data on long-term outcomes are lacking with Medicare data suggesting that TEVAR has worse late survival compared with open descending repair. As such, the purpose of this study was to examine the long-term outcomes for on-label use of TEVAR for repair of descending thoracic aneurysms.
Methods: Of 579 patients undergoing TEVAR between March 2005 and April 2016 at a single referral center for aortic surgery, 192 (33.2%) were performed for a descending thoracic aneurysm indication in accordance with the device instructions for use, including 106 fusiform (55.2%), 80 saccular (41.7%), and 6 with both saccular and fusiform (3.1%) aneurysms. All aneurysms were located distal to the left subclavian artery and proximal to the celiac axis, and hybrid procedures including arch or visceral debranching were excluded with the exception of left carotid-subclavian artery bypass. Aortic dissection and intramural hematoma as indications for TEVAR were also excluded. Primary 30-day and in-hospital outcomes included mortality, stroke, need for new permanent dialysis, and permanent paraparesis or paraplegia. Primary long-term outcomes included survival and rate of reintervention secondary to endoleak. The Kaplan-Meier method was used to estimate long-term overall and aorta-specific survivals.
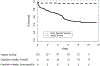
Results: The mean age was 71.1 ± 10.4 years. All aneurysms in this series were degenerative in nature and no patients with a connective tissue disorder were included. The mean aortic diameter was 5.9 ± 1.5 cm at time of intervention. Rates of 30-day and in-hospital mortality, stroke, permanent dialysis, and permanent paraparesis and paraplegia were 4.7%, 2.1%, 0.5%, and 0.5%, respectively. At a mean follow-up of 69 ± 44 months (range, 3-141 months), there were 68 late deaths (35.4%), two of which were due to aortic rupture. Overall and aorta-specific survivals at 141 months (11.8 years) were 45.7% and 96.2%, respectively. Endovascular reintervention was required in 14 patients (7.3%) owing to type I (n = 10), type II (n = 2), and type III (n = 2) endoleak, all of which subsequently resolved. No patient required open reintervention for any cause.
Conclusions: Long-term (12-year) aorta-specific survival after on-label endovascular repair of degenerative descending thoracic aneurysms in nonsyndromic patients is excellent (96%) with sustained protection from rupture, and a low rate of reintervention owing to endoleak (7%). Endovascular repair should be considered the treatment of choice for this pathology.
Copyright © 2017 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Author conflict of interest: none.
Figures
References
-
- Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1–9. - PubMed
-
- Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369–77. - PubMed
-
- Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg 2008;47:912–8. - PubMed
-
- Patterson BO, Thompson MM. The MOTHER Multicenter Registry: an overview and discussion of a registry that may help us understand how and to what degree various factors influence the risk of complications after TEVAR. Endovascular Today 2012:10–4.
-
- Leurs LJ, Bell R, Degrieck Y, Thomas S, Hobo R, Lundbom J. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg 2004;40:670–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous