Candida albicans and Pseudomonas aeruginosa Interact To Enhance Virulence of Mucosal Infection in Transparent Zebrafish
- PMID: 28847848
- PMCID: PMC5649025
- DOI: 10.1128/IAI.00475-17
Candida albicans and Pseudomonas aeruginosa Interact To Enhance Virulence of Mucosal Infection in Transparent Zebrafish
Abstract
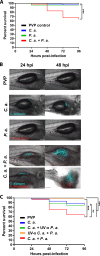
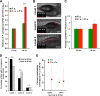
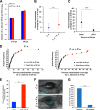
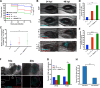
Polymicrobial infections often include both fungi and bacteria and can complicate patient treatment and resolution of infection. Cross-kingdom interactions among bacteria, fungi, and/or the immune system during infection can enhance or block virulence mechanisms and influence disease progression. The fungus Candida albicans and the bacterium Pseudomonas aeruginosa are coisolated in the context of polymicrobial infection at a variety of sites throughout the body, including mucosal tissues such as the lung. In vitro, C. albicans and P. aeruginosa have a bidirectional and largely antagonistic relationship. Their interactions in vivo remain poorly understood, specifically regarding host responses in mediating infection. In this study, we examine trikingdom interactions using a transparent juvenile zebrafish to model mucosal lung infection and show that C. albicans and P. aeruginosa are synergistically virulent. We find that high C. albicans burden, fungal epithelial invasion, swimbladder edema, and epithelial extrusion events serve as predictive factors for mortality in our infection model. Longitudinal analyses of fungal, bacterial, and immune dynamics during coinfection suggest that enhanced morbidity is associated with exacerbated C. albicans pathogenesis and elevated inflammation. The P. aeruginosa quorum-sensing-deficient ΔlasR mutant also enhances C. albicans pathogenicity in coinfection and induces extrusion of the swimbladder. Together, these observations suggest that C. albicans-P. aeruginosa cross talk in vivo can benefit both organisms to the detriment of the host.
Keywords: Candida albicans; Pseudomonas aeruginosa; infection; mucosal; polymicrobial; zebrafish.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Kim SH, Clark ST, Surendra A, Copeland JK, Wang PW, Ammar R, Collins C, Tullis DE, Nislow C, Hwang DM, Guttman DS, Cowen LE. 2015. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog 11:e1005308. doi:10.1371/journal.ppat.1005308. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

