Dendritic cell and antigen dispersal landscapes regulate T cell immunity
- PMID: 28847868
- PMCID: PMC5626399
- DOI: 10.1084/jem.20170335
Dendritic cell and antigen dispersal landscapes regulate T cell immunity
Abstract
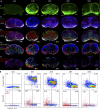
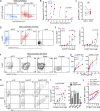
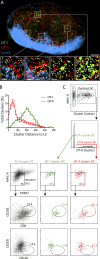
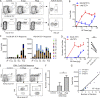
Dendritic cell (DC) subsets with biased capacity for CD4+ and CD8+ T cell activation are asymmetrically distributed in lymph nodes (LNs), but how this affects adaptive responses has not been extensively studied. Here we used quantitative imaging to examine the relationships among antigen dispersal, DC positioning, and T cell activation after protein immunization. Antigens rapidly drained into LNs and formed gradients extending from the lymphatic sinuses, with reduced abundance in the deep LN paracortex. Differential localization of DCs specialized for major histocompatibility complex I (MHC I) and MHC II presentation resulted in preferential activation of CD8+ and CD4+ T cells within distinct LN regions. Because MHC I-specialized DCs are positioned in regions with limited antigen delivery, modest reductions in antigen dose led to a substantially greater decline in CD8+ compared with CD4+ T cell activation, expansion, and clonal diversity. Thus, the collective action of antigen dispersal and DC positioning regulates the extent and quality of T cell immunity, with important implications for vaccine design.
© 2017 Gerner et al.
Figures

References
-
- Baekkevold E.S., Yamanaka T., Palframan R.T., Carlsen H.S., Reinholt F.P., von Andrian U.H., Brandtzaeg P., and Haraldsen G.. 2001. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193:1105–1112. 10.1084/jem.193.9.1105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

