Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow
- PMID: 28847942
- PMCID: PMC5604020
- DOI: 10.1073/pnas.1706942114
Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow
Abstract
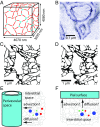
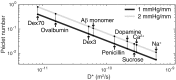
The brain lacks lymph vessels and must rely on other mechanisms for clearance of waste products, including amyloid [Formula: see text] that may form pathological aggregates if not effectively cleared. It has been proposed that flow of interstitial fluid through the brain's interstitial space provides a mechanism for waste clearance. Here we compute the permeability and simulate pressure-mediated bulk flow through 3D electron microscope (EM) reconstructions of interstitial space. The space was divided into sheets (i.e., space between two parallel membranes) and tunnels (where three or more membranes meet). Simulation results indicate that even for larger extracellular volume fractions than what is reported for sleep and for geometries with a high tunnel volume fraction, the permeability was too low to allow for any substantial bulk flow at physiological hydrostatic pressure gradients. For two different geometries with the same extracellular volume fraction the geometry with the most tunnel volume had [Formula: see text] higher permeability, but the bulk flow was still insignificant. These simulation results suggest that even large molecule solutes would be more easily cleared from the brain interstitium by diffusion than by bulk flow. Thus, diffusion within the interstitial space combined with advection along vessels is likely to substitute for the lymphatic drainage system in other organs.
Keywords: AQP4; extracellular space; glymphatic; interstitial fluid; simulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Thrane AS, Rangroo Thrane V, Plog BA, Nedergaard M. Filtering the muddied waters of brain edema. Trends Neurosci. 2015;38:333–335. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

