The P2X7 Receptor Primes IL-1β and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain
- PMID: 28848393
- PMCID: PMC5550720
- DOI: 10.3389/fncel.2017.00227
The P2X7 Receptor Primes IL-1β and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain
Abstract
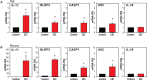
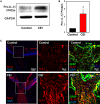
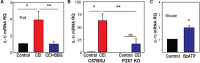
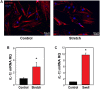
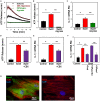
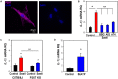
Inflammatory responses play a key role in many neural pathologies, with localized signaling from the non-immune cells making critical contributions. The NLRP3 inflammasome is an important component of innate immune signaling and can link neural insult to chronic inflammation. The NLRP3 inflammasome requires two stages to contribute: priming and activation. The priming stage involves upregulation of inflammasome components while the activation stage results in the assembly and activation of the inflammasome complex. The priming step can be rate limiting and can connect insult to chronic inflammation, but our knowledge of the signals that regulate NLRP3 inflammasome priming in sterile inflammation is limited. This study examined the link between mechanical strain and inflammasome priming in neural systems. Transient non-ischemic elevation of intraocular pressure increased mRNA for inflammasome components IL-1β, NLRP3, ASC, and CASP1 in rat and mouse retinas. The elevation was greater 1 day after the insult, with the rise in IL-1β most pronounced. The P2X7 receptor was implicated in the mechanosensitive priming of IL-1β mRNA in vivo, as the antagonist Brilliant Blue G (BBG) blocked the increased expression, the agonist BzATP mimicked the pressure-dependent rise in IL-1β, and the rise was absent in P2X7 knockout mice. In vitro measurements from optic nerve head astrocytes demonstrated an increased expression of IL-1β following stretch or swelling. This increase in IL-1β was eliminated by degradation of extracellular ATP with apyrase, or by the block of pannexin hemichannels with carbenoxolone, probenecid, or 10panx1 peptide. The rise in IL-1β expression was also blocked by P2X7 receptor antagonists BBG, A839977 or A740003. The rise in IL-1β was prevented by blocking transcription factor NFκB with Bay 11-7082, while the swelling-dependent fall in NFκB inhibitor IκB-α was reduced by A839977 and in P2X7 knockout mice. In summary, mechanical trauma to the retina primed NLRP3 inflammasome components, but only if there was ATP release through pannexin hemichannels, and autostimulation of the P2X7 receptor. As the P2X7 receptor can also trigger stage two of inflammasome assembly and activation, the P2X7 receptor may have a central role in linking mechanical strain to neuroinflammation.
Keywords: ATP release; IL-1β; NFκB; NLRP3; astrocytes; caspase 1; glaucoma; pannexin.
Figures


References
-
- Albalawi F., Lu W., Lim J., Mitchell C. H. (2016). The role of P2X7R in priming the NLRP3 inflammasome after mechanical strain. FASEB J. 30 745.
-
- Beckel J. M., Argall A. J., Lim J. C., Xia J., Lu W., Coffey E. E., et al. (2014). Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62 1486–1501. 10.1002/glia.22695 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

