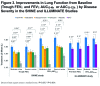
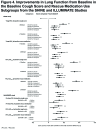
QVA149 Improves Lung Function, Dyspnea, and Health Status Independent of Previously Prescribed Medications and COPD Severity: A Subgroup Analysis from the SHINE and ILLUMINATE Studies
- PMID: 28848830
- PMCID: PMC5556773
- DOI: 10.15326/jcopdf.2.1.2014.0140
QVA149 Improves Lung Function, Dyspnea, and Health Status Independent of Previously Prescribed Medications and COPD Severity: A Subgroup Analysis from the SHINE and ILLUMINATE Studies
Abstract
Background: QVA149 is a dual bronchodilator combining the long-acting β2-agonist(LABA) indacaterol and the long-acting muscarinic antagonist (LAMA) glycopyrronium, for maintenance treatment of COPD. This post hoc analysis evaluated the improvements in lung function, dyspnea, and health status in subgroups of patients based on prior medication use, disease severities, baseline cough score, and baseline rescue medication use, achieved with QVA149 compared with placebo and other active comparators in 2 phase III clinical studies. Methods: In both the SHINE (NCT01202188) and ILLUMINATE (NCT01315249) studies, symptomatic patients aged ≥40 years with moderate-to-severe COPD were randomized to once-daily QVA149 (110/50 µg), indacaterol (150 µg), glycopyrronium (50 µg), tiotropium (18 μg), or placebo (2:2:2:2:1) and once-daily QVA149 (110/50 µg) or twice-daily salmeterol/fluticasone ([SFC]; 50/500 µg), respectively for 26 weeks. Here, we present the improvements in lung function, transition dyspnea index (TDI) and St. George's Respiratory Questionnaire (SGRQ) total score by prior medication use and COPD disease severity separately from both studies. Results: In total, 2144 and 523 patients were randomized in the SHINE and ILLUMINATE studies; 89.1% and 82.6%, respectively, completed the study. QVA149 showed significant improvements in lung function compared with placebo (SHINE study) and SFC (ILLUMINATE study) regardless of prior medication, disease severity, baseline cough score, and rescue medication use. TDI and SGRQ total scores were also improved with QVA149 compared with placebo and SFC in most of the analyzed subgroups. Conclusion: QVA149 showed improvements in lung function, dyspnea, and health status in both moderate and severe COPD patients independent of previous medication use and baseline cough score.
Keywords: QVA149; combination therapy; disease severity; prior medication; subgroups.
Conflict of interest statement
KRC, in the last 3 years, has received compensation for consulting with Almirall, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck Frosst, Novartis, Takeda, Pfizer, Roche, Schering Plough and Grifols; has undertaken research funded by AstraZeneca, Boehringer Ingelheim, CSL Behring, Forest Labs, GlaxoSmithKline, Novartis, Roche, Takeda and Grifols; and has participated in continuing medical education activities sponsored in whole or in part by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, Merck Frosst, Novartis, Takeda and Pfizer. He is participating in research funded by the Canadian Institutes of Health Research operating grant entitled Canadian Cohort Obstructive Lung Disease (CanCOLD). He holds the GSK-CIHR Research Chair in Respiratory Health Care Delivery at the University Health Network, Toronto, Canada. EDB has served as a consultant to AlkAbello, Almirall, Cephalon, Hoffman la Roche, ICON and MS Consulting Group; been on advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim, Elevation Pharma, Forest, GlaxoSmithKline, Merck, Napp, Novartis and Nycomed; and received lecture fees from AlkAbello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer and Takeda; and his institution has received remuneration for participation in clinical trials sponsored by Actelion, Aeras, Almirall, AstraZeneca, Boehringer Ingelheim, Forest, GlaxoSmithKline, Hoffman La Roche, Merck, Novartis, Takeda and TEVA. HC,HH, RF and DB are employees of Novartis and have no other conflicts of interest.
References
-
- Baloira A. Which is the optimal bronchodilator therapy for chronic obstructive pulmonary disease?. Expert Rev Respir Med. 2013;7(2s):17-24. doi: http://dx.doi.org/10.1586/ers.13.18 - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2014http://www.goldcopd.com Updated January 2014 Accessed February 18, 2014. - PubMed
-
- Van Noord JA,Aumann JL,Janssens E,et al. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respir Med. 2010;104(7):995-1004. doi: http://dx.doi.org/10.1016/j.rmed.2010.02.017 - PubMed
-
- Vogelmeier C,Kardos P,Harari S,et al. Formoterol mono-and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008;102(11):1511-1520. doi: http://dx.doi.org/10.1016/j.rmed.2008.07.020 - PubMed
-
- Cazzola M,Page CP,Calzetta L,et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450-504. doi: http://dx.doi.org/10.1124/pr.111.004580 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources