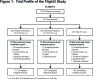
Long-Term Maintenance Bronchodilation With Indacaterol/Glycopyrrolate Versus Indacaterol in Moderate-to-Severe COPD Patients: The FLIGHT 3 Study
- PMID: 28848898
- PMCID: PMC5556955
- DOI: 10.15326/jcopdf.3.4.2016.0131
Long-Term Maintenance Bronchodilation With Indacaterol/Glycopyrrolate Versus Indacaterol in Moderate-to-Severe COPD Patients: The FLIGHT 3 Study
Abstract
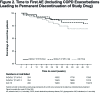
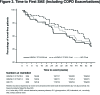
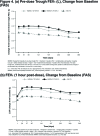
Background: The objective of the FLIGHT3 study was to evaluate the long-term safety and efficacy of indacaterol/glycopyrrolate* (IND/GLY) versus an active comparator, IND, in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD) over 52 weeks. Method: FLIGHT3 was a multicenter, randomized, double-blind, parallel-group, 52-week study. Patients were randomized (1:1:1) to IND/GLY (27.5/15.6 or 27.5/31.2 µg twice daily [b.i.d.]) or IND (75 µg once daily [o.d.]), delivered via the Neohaler® device. The primary objective was to evaluate the long-term safety and tolerability of IND/GLY versus IND in terms of adverse event (AE)-reporting rates in patients with moderate-to-severe COPD over 52 weeks. The secondary objective was to evaluate the long-term efficacy of IND/GLY versus IND in terms of pre-dose trough forced expiratory volume in 1 second (FEV1) and post-dose 1-h FEV1 over 52 weeks. Results: A total of 85.2% patients completed the study treatment. The overall incidence of AEs (and SAEs) was similar between treatments. Major adverse cardiovascular events (MACE) and/or cardiovascular (CV) events were comparable between treatment groups. The rate of discontinuation of the study treatment due to AEs was lower for IND/GLY than IND. Improvements in pre-dose trough FEV1 and post-dose 1-h FEV1 were consistently superior with IND/GLY than with IND over 52 weeks, demonstrating long-term maintenance of lung function. Conclusions: IND/GLY demonstrated a favorable long-term safety and tolerability profile and provided effective bronchodilation, with maintenance of lung function over 52 weeks in patients with moderate-to-severe COPD. These data support the safety and efficacy of IND/GLY as a treatment option for COPD. Trial registration: ClinTrials.gov identifier NCT01682863 *Glycopyrrolate 15.6 µg (excluding the bromide salt) is equivalent to 12.5 µg glycopyrronium.
Keywords: chronic obstructive pulmonary disease; clinical trials; copd; long-acting beta2-agonists; long-acting muscarinic antagonist.
Conflict of interest statement
GF is supported in part by multiple pharmaceutical clinical research projects, including grants from Novartis Pharmaceuticals, Corp. AFT, AST, FP and DB are employees of Novartis Pharmaceuticals Corp.
References
-
- Global initiative for chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD website. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_.pdf Published 2015 Accessed May 3, 2015.
-
- Blasi F,Cesana G,Conti S,et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PloS One. 2014;9(6): e101228 doi: http://dx.doi.org/10.1371/journal.pone.0101228 - PMC - PubMed
-
- Miravitlles M,Sicras A,Crespo C,et al. Costs of chronic obstructive pulmonary disease in relation to compliance with guidelines: a study in the primary care setting. Ther Adv Respir Dis. 2013;7(3):139-150. doi: http://dx.doi.org/10.1177/1753465813484080 - PubMed
-
- Rutten-van Molken MP,Goossens LM. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease: a review of the evidence and methodological issues . Pharmacoeconomics. 2012;30(4):271-302. doi: http://dx.doi.org/10.2165/11589270-000000000-00000 - PubMed
-
- National Institutes of Health; National Heart Lung and Blood Institute (NHLBI). NHLBI Chartbook 2012. NHLBI website. http://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf Published February 2012 Accessed April 21, 2015.
LinkOut - more resources
Full Text Sources
Other Literature Sources