Prevalence of Low Peak Inspiratory Flow Rate at Discharge in Patients Hospitalized for COPD Exacerbation
- PMID: 28848933
- PMCID: PMC5556913
- DOI: 10.15326/jcopdf.4.3.2017.0183
Prevalence of Low Peak Inspiratory Flow Rate at Discharge in Patients Hospitalized for COPD Exacerbation
Abstract
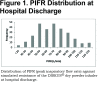
Background: Low peak inspiratory flow rate (PIFR) (<60 L/min) among patients with chronic obstructive pulmonary disease (COPD) may result in ineffective medication inhalation, leading to poor bronchodilation. Objective: The objectives of this analysis were to evaluate the prevalence of low PIFR at the time of discharge from a COPD-related hospitalization and to examine the real-world treatment patterns and rehospitalizations by PIFR. Methods: Patients at 7 sites in the United States were screened for enrollment at hospital discharge. PIFR was measured using the InCheckTM DIAL to simulate resistance of the DISKUS® dry powder inhaler (DPI). An equal number of patients were enrolled into low PIFR (<60 L/min) or normal PIFR (≥60 L/min) cohorts. Demographics, COPD-related clinical characteristics, health status, treatment and rehospitalization data were collected. Results: Mean PIFR was 71±22.12 L/min among 268 screened patients; 31.7% (n=85) of patients had low PIFR. Among all enrolled patients (n=170), the low PIFR cohort was older (66.2±10.04 years versus 62.1±9.41 years, p=0.006) and more likely to be female (61.2% versus 42.4%, p=0.014). There was an increase in DPI use at discharge, compared with admission, in the low PIFR cohort (62.4% versus 70.6%, p=0.020). The incidences of all-cause rehospitalization up to 180 days were similar between the low and normal PIFR cohorts. Conclusions: At discharge following hospitalization for an exacerbation of COPD, approximately one-third of patients had a PIFR <60 L/min. More patients with a low PIFR were discharged with a DPI medication compared with use at admission. There was no difference in the rehospitalization rates by PIFR.
Keywords: PIFR; chronic obstructive pulmonary disease; copd; discharge planning; exacerbation; outcomes research; peak inspiratory flow rate.
Conflict of interest statement
Gulshan Sharma serves on the scientific advisory board at Sunovion Pharmaceuticals, Inc. Donald A. Mahler serves on an advisory board and a speaker’s bureau for Sunovion Pharmaceuticals, Inc. Valerie M. Mayorga, Kathleen L. Deering, and Qing Harshaw are employed at EPI-Q, Inc., which received funding from Sunovion Pharmaceuticals, Inc., for this analysis. Vaidyanathan Ganapathy is a full-time employee of Sunovion Pharmaceuticals, Inc. The content of this manuscript, the ultimate interpretation, and the decision to submit for publication was made by the authors independently.
References
-
- Centers for Disease Control and Prevention; National Center for Health Statistics. Deaths: Final Data for 2013. Nat Vital Stat Rep. 2016;64(2):1-119. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease; Global Strategy for the Diagnosis, Management and Prevention of COPD, 2016. GOLD website. http://goldcopd.org/ Published 2016 Accessed July 18, 2016.
-
- Pauwels R,Newman S,Borgstrom L. Airway deposition and airway effects of antiasthma drugs delivered from metered-dose inhalers. Eur Respir J. 1997;10(9):2127-2138. doi: https://doi.org/10.1183/09031936.97.10092127 - PubMed
-
- Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50(9):1177-1190. - PubMed
-
- Newman SP. Drug delivery to the lungs from dry powder inhalers. Curr Opin Pulm Med. 2003;9(suppl1):S17-20. doi: https://doi.org/10.1097/00063198-200304001-00005 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources