Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma
- PMID: 28849310
- PMCID: PMC5700237
- DOI: 10.1007/s11060-017-2601-y
Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma
Abstract
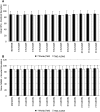
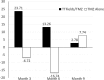
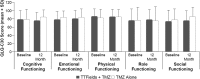
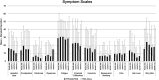
We characterized health-related quality of life (HRQoL), cognitive, and functional status in newly diagnosed glioblastoma (GBM) patients receiving Tumor treating fields (TTFields) with temozolomide (TMZ) versus TMZ alone in a planned interim analysis of a randomized phase III trial [NCT00916409], which showed significant improvement in progression-free and overall survival with TTFields/TMZ. After radiotherapy with concomitant TMZ, newly diagnosed GBM patients were randomized (2:1) to TTFields/TMZ (n = 210) or TMZ (n = 105). Interim analysis was performed in 315 patients with ≥18 months of follow-up. HRQoL, a secondary endpoint, was evaluated in per-protocol patient population and expressed as change from baseline (CFB) at 3, 6, and 9 months for each subscale in the EORTC QLQ-C30/BN20. Karnofsky performance scores (KPS) and Mini-Mental State Examination scores (MMSE) were assessed. CFB in HRQoL was balanced in treatment groups at the 12-month time point. Initially, HRQoL improved in patients treated with TTFields/TMZ (CFB3: 24% and CFB6: 13%) versus TMZ (CFB3: -7% and CFB6: -17%), though this difference was no longer evident at the 9-month point. General scales, including physical and social functioning, showed no difference at 9 and 12 months. TTFields/TMZ group reported higher concerns of "itchy skin". KPS over 12 months was just below 90 in both groups. Cognitive status (MMSE) was stable over time. HRQoL, KPS, and MMSE were balanced in both groups over time. There was no preliminary evidence that HRQoL, cognitive, and functional status is adversely affected by the continuous use of TTFields.
Keywords: Cognition; EF-14; Glioblastoma; Health-related quality of life (HRQoL); Temozolomide; Tumor treating fields.
Conflict of interest statement
Jay-Jiguang Zhu, MD, PhD has disclosed that he has received research funding from Novocure Inc., Five PRIME, Inc. Immuno-Cellular Inc., Diffusion Pharmaceuticals LLC, and DEKK-TEC, Inc. Petya Demireva, PhD, declares that she has no conflict of interest. Andrew Kanner, MD, declares that he has no conflict of interest. Susan Pannullo, MD, declares she has served as a consultant or participated in an advisory board for Cancer Panels, Guidepoint Global, Clearview Healthcare Partners, ICF International and MAPI; has received research funding from Novocure, Celldex, Immunocellular Therapeutics, and Stemline, and holds a patent from Arrow Development Corp; and has received reimbursement for travel from Cancer Panels. Maximilian Mehdorn, MD, has declared he served as a consultant or participated in an advisory board for Stryker Leibinger. Nicholas Avgeropoulos, MD, has declared he received honoraria from and participated in a speakers bureau for Novocure. Andrea Salmaggi, MD, has provided expert testimony from UCB and has received travel reimbursement from Italfarmaco. Antonio Silvani, MD, has declared he received honoraria and research funding from Novocure. Samuel Goldlust, MD, declares that he has no conflict of interest. Carlos David, MD, declares he owns stock in Kogent Surgical. Alexandra Benouaich-Amiel, MD, declares receiving research funding from Roche. Zvi Ram, MD, has declared owning stock in Novocure and receiving honoraria from Novocure and Research to Practice Meeting, and has received travel reimbursement from Novocure, BrainLAB, and Electra Pharmaceuticals.
Figures

References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

