The Treponema pallidum Outer Membrane
- PMID: 28849315
- PMCID: PMC5924592
- DOI: 10.1007/82_2017_44
The Treponema pallidum Outer Membrane
Abstract
The outer membrane (OM) of Treponema pallidum, the uncultivatable agent of venereal syphilis, has long been the subject of misconceptions and controversy. Decades ago, researchers postulated that T. pallidum's poor surface antigenicity is the basis for its ability to cause persistent infection, but they mistakenly attributed this enigmatic property to the presence of a protective outer coat of serum proteins and mucopolysaccharides. Subsequent studies revealed that the OM is the barrier to antibody binding, that it contains a paucity of integral membrane proteins, and that the preponderance of the spirochete's immunogenic lipoproteins is periplasmic. Since the advent of recombinant DNA technology, the fragility of the OM, its low protein content, and the lack of sequence relatedness between T. pallidum and Gram-negative outer membrane proteins (OMPs) have complicated efforts to characterize molecules residing at the host-pathogen interface. We have overcome these hurdles using the genomic sequence in concert with computational tools to identify proteins predicted to form β-barrels, the hallmark conformation of OMPs in double-membrane organisms and evolutionarily related eukaryotic organelles. We also have employed diverse methodologies to confirm that some candidate OMPs do, in fact, form amphiphilic β-barrels and are surface-exposed in T. pallidum. These studies have led to a structural homology model for BamA and established the bipartite topology of the T. pallidum repeat (Tpr) family of proteins. Recent bioinformatics has identified several structural orthologs for well-characterized Gram-negative OMPs, suggesting that the T. pallidum OMP repertoire is more Gram-negative-like than previously supposed. Lipoprotein adhesins and proteases on the spirochete surface also may contribute to disease pathogenesis and protective immunity.
Figures




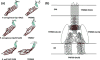
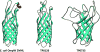
References
-
- Anand A, LeDoyt M, Karanian C, Luthra A, Koszelak-Rosenblum M, Malkowski MG, Puthenveetil R, Vinogradova O, Radolf JD. Bipartite topology of Treponema pallidum repeat proteins C/D and I: outer membrane insertion, trimerization, and porin function require a C-terminal β-barrel domain. J Biol Chem. 2015;290:12313–12331. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

