Comprehensive Analyses of Tissue-Specific Networks with Implications to Psychiatric Diseases
- PMID: 28849569
- PMCID: PMC5699888
- DOI: 10.1007/978-1-4939-7027-8_15
Comprehensive Analyses of Tissue-Specific Networks with Implications to Psychiatric Diseases
Abstract
Recent advances in genome sequencing and "omics" technologies are opening new opportunities for improving diagnosis and treatment of human diseases. The precision medicine initiative in particular aims at developing individualized treatment options that take into account individual variability in genes and environment of each person. Systems biology approaches that group genes, transcripts and proteins into functionally meaningful networks will play crucial role in the future of personalized medicine. They will allow comparison of healthy and disease-affected tissues and organs from the same individual, as well as between healthy and disease-afflicted individuals. However, the field faces a multitude of challenges ranging from data integration to statistical and combinatorial issues in data analyses. This chapter describes computational approaches developed by us and the others to tackle challenges in tissue-specific network analyses, with the main focus on psychiatric diseases.
Keywords: Alternatively spliced isoforms; Autism; Copy number variants; De novo mutations; Gene expression; Genetics; Network analyses; Protein–protein interactions; Psychiatric diseases; Systems biology.
Figures
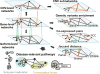





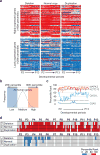



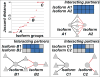

References
-
- Parikshak NN, Gandal MJ, Geschwind DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet. 2015;16(8):441–458. doi: 10.1038/nrg3934. http://www.nature.com/nrg/journal/v16/n8/abs/nrg3934.html#supplementary-.... - DOI - PMC - PubMed
-
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. nature10523 [pii] - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

