Magnetic Nanoparticles: From Design and Synthesis to Real World Applications
- PMID: 28850089
- PMCID: PMC5618354
- DOI: 10.3390/nano7090243
Magnetic Nanoparticles: From Design and Synthesis to Real World Applications
Abstract
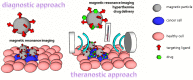
The increasing number of scientific publications focusing on magnetic materials indicates growing interest in the broader scientific community. Substantial progress was made in the synthesis of magnetic materials of desired size, morphology, chemical composition, and surface chemistry. Physical and chemical stability of magnetic materials is acquired by the coating. Moreover, surface layers of polymers, silica, biomolecules, etc. can be designed to obtain affinity to target molecules. The combination of the ability to respond to the external magnetic field and the rich possibilities of coatings makes magnetic materials universal tool for magnetic separations of small molecules, biomolecules and cells. In the biomedical field, magnetic particles and magnetic composites are utilized as the drug carriers, as contrast agents for magnetic resonance imaging (MRI), and in magnetic hyperthermia. However, the multifunctional magnetic particles enabling the diagnosis and therapy at the same time are emerging. The presented review article summarizes the findings regarding the design and synthesis of magnetic materials focused on biomedical applications. We highlight the utilization of magnetic materials in separation/preconcentration of various molecules and cells, and their use in diagnosis and therapy.
Keywords: magnetic resonance imaging; nanocarrier; nanoscale; preconcentration; separation; silica; theranostics; therapeutic agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pankhurst Q.A., Connolly J., Jones S.K., Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003;36:167–181. doi: 10.1088/0022-3727/36/13/201. - DOI
-
- Bessalova V., Perov N., Rodionova V. New approaches in the design of magnetic tweezers-current magnetic tweezers. J. Magn. Magn. Mater. 2016;415:66–71. doi: 10.1016/j.jmmm.2016.03.038. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

