Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: A hospital-based cohort study
- PMID: 28850568
- PMCID: PMC5574534
- DOI: 10.1371/journal.pmed.1002379
Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: A hospital-based cohort study
Abstract
Background: Primaquine is the only licensed drug for eradicating Plasmodium vivax hypnozoites and, therefore, preventing relapses of vivax malaria. It is a vital component of global malaria elimination efforts. Primaquine is efficacious when supervised in clinical trials, but its effectiveness in real-world settings is unknown. We aimed to determine whether unsupervised primaquine was effective for preventing re-presentation to hospital with vivax malaria in southern Papua, Indonesia.
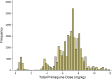
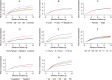
Methods and findings: Routinely-collected hospital surveillance data were used to undertake a pragmatic comparison of the risk of re-presentation to hospital with vivax malaria in patients prescribed dihydroartemisinin-piperaquine (DHP) combined with primaquine versus those patients prescribed DHP alone. The omission of primaquine was predominantly due to 3 stock outages. Individual clinical, pharmacy, and laboratory data were merged using individual hospital identification numbers and the date of presentation to hospital. Between April 2004 and December 2013, there were 86,797 documented episodes of vivax malaria, of which 62,492 (72.0%) were included in the analysis. The risk of re-presentation with vivax malaria within 1 year was 33.8% (95% confidence Interval [CI] 33.1%-34.5%) after initial monoinfection with P. vivax and 29.2% (95% CI 28.1%-30.4%) after mixed-species infection. The risk of re-presentation with P. vivax malaria was higher in children 1 to <5 years of age (49.6% [95% CI 48.4%-50.9%]) compared to patients 15 years of age or older (24.2% [95% CI 23.4-24.9%]); Adjusted Hazard Ratio (AHR) = 2.23 (95% CI 2.15-2.31), p < 0.001. Overall, the risk of re-presentation was 37.2% (95% CI 35.6%-38.8%) in patients who were prescribed no primaquine compared to 31.6% (95% CI 30.9%-32.3%) in those prescribed either a low (≥1.5 mg/kg and <5 mg/kg) or high (≥5 mg/kg) dose of primaquine (AHR = 0.90 [95% CI 0.86-0.95, p < 0.001]). Limiting the comparison to high dose versus no primaquine in the period during and 12 months before and after a large stock outage resulted in minimal change in the estimated clinical effectiveness of primaquine (AHR 0.91, 95% CI 0.85-0.97, p = 0.003). Our pragmatic study avoided the clinical influences associated with prospective study involvement but was subject to attrition bias caused by passive follow-up.
Conclusions: Unsupervised primaquine for vivax malaria, prescribed according to the current World Health Organization guidelines, was associated with a minimal reduction in the risk of clinical recurrence within 1 year in Papua, Indonesia. New strategies for the effective radical cure of vivax malaria are needed in resource-poor settings.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Thriemer K, Ley B, Bobogare A, Dysoley L, Alam MS, Pasaribu AP, et al. Challenges for achieving safe and effective radical cure of Plasmodium vivax: a round table discussion of the APMEN Vivax Working Group. Malar J. 2017;16(1):141 doi: 10.1186/s12936-017-1784-1 - DOI - PMC - PubMed
-
- World Health Organization. Guidelines for the treatment of malaria - 3rd edition Geneva: World Health Organization; 2015.
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–45. doi: 10.1086/424663 - DOI - PubMed
-
- Maneeboonyang W, Lawpoolsri S, Puangsa-Art S, Yimsamran S, Thanyavanich N, Wuthisen P, et al. Directly observed therapy with primaquine to reduce the recurrence rate of Plasmodium vivax infection along the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2011;42(1):9–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

