Structural studies demonstrating a bacteriophage-like replication cycle of the eukaryote-infecting Paramecium bursaria chlorella virus-1
- PMID: 28850602
- PMCID: PMC5593192
- DOI: 10.1371/journal.ppat.1006562
Structural studies demonstrating a bacteriophage-like replication cycle of the eukaryote-infecting Paramecium bursaria chlorella virus-1
Abstract
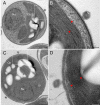
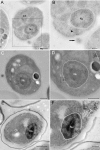
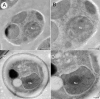
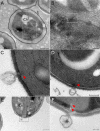
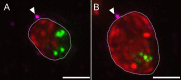
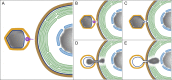
A fundamental stage in viral infection is the internalization of viral genomes in host cells. Although extensively studied, the mechanisms and factors responsible for the genome internalization process remain poorly understood. Here we report our observations, derived from diverse imaging methods on genome internalization of the large dsDNA Paramecium bursaria chlorella virus-1 (PBCV-1). Our studies reveal that early infection stages of this eukaryotic-infecting virus occurs by a bacteriophage-like pathway, whereby PBCV-1 generates a hole in the host cell wall and ejects its dsDNA genome in a linear, base-pair-by-base-pair process, through a membrane tunnel generated by the fusion of the virus internal membrane with the host membrane. Furthermore, our results imply that PBCV-1 DNA condensation that occurs shortly after infection probably plays a role in genome internalization, as hypothesized for the infection of some bacteriophages. The subsequent perforation of the host photosynthetic membranes presumably enables trafficking of viral genomes towards host nuclei. Previous studies established that at late infection stages PBCV-1 generates cytoplasmic organelles, termed viral factories, where viral assembly takes place, a feature characteristic of many large dsDNA viruses that infect eukaryotic organisms. PBCV-1 thus appears to combine a bacteriophage-like mechanism during early infection stages with a eukaryotic-like infection pathway in its late replication cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320: 531–535. doi: 10.1126/science.1155164 - DOI - PubMed
-
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nature Cell Biol. 2009;11: 510–520. doi: 10.1038/ncb0509-510 - DOI - PubMed
-
- Molineux IJ. Fifty-three years since Hershey and Chase; much ado about pressure but which pressure is it? Virology. 2006;344: 221–229. doi: 10.1016/j.virol.2005.09.014 - DOI - PubMed
-
- Molineux IJ, Panja D. Popping the cork: mechanisms of phage genome ejection. Nature Rev Microbiol. 2013;11: 194–204. - PubMed
-
- Iyer LA, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006;117: 156–184. doi: 10.1016/j.virusres.2006.01.009 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

