Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects
- PMID: 28851217
- PMCID: PMC5673097
- DOI: 10.1021/jacs.7b07311
Mechanism of Nitrogenase H2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects
Abstract
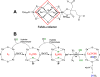
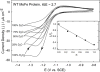
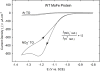
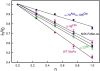
Nitrogenase catalyzes the reduction of dinitrogen (N2) to two ammonia (NH3) at its active site FeMo-cofactor through a mechanism involving reductive elimination of two [Fe-H-Fe] bridging hydrides to make H2. A competing reaction is the protonation of the hydride [Fe-H-Fe] to make H2. The overall nitrogenase rate-limiting step is associated with ATP-driven electron delivery from Fe protein, precluding isotope effect measurements on substrate reduction steps. Here, we use mediated bioelectrocatalysis to drive electron delivery to the MoFe protein allowing examination of the mechanism of H2 formation by the metal-hydride protonation reaction. The ratio of catalytic current in mixtures of H2O and D2O, the proton inventory, was found to change linearly with the D2O/H2O ratio, revealing that a single H/D is involved in the rate-limiting step of H2 formation. Kinetic models, along with measurements that vary the electron/proton delivery rate and use different substrates, reveal that the rate-limiting step under these conditions is the H2 formation reaction. Altering the chemical environment around the active site FeMo-cofactor in the MoFe protein, either by substituting nearby amino acids or transferring the isolated FeMo-cofactor into a different peptide matrix, changes the net isotope effect, but the proton inventory plot remains linear, consistent with an unchanging rate-limiting step. Density functional theory predicts a transition state for H2 formation where the S-H+ bond breaks and H+ attacks the Fe-hydride, and explains the observed H/D isotope effect. This study not only reveals the nitrogenase mechanism of H2 formation by hydride protonation, but also illustrates a strategy for mechanistic study that can be applied to other oxidoreductase enzymes and to biomimetic complexes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Smil V. Enriching the earth: Fritz Haber, Carl Bosch, and the transformation of world food production. MIT Press; Cambridge, Mass: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

