Precisão e confiabilidade de um teste imuno-cromatográfico rápido NS1 para diagnóstico DENV-1 no ponto de atendimento e no laboratório
- PMID: 28851293
- PMCID: PMC5576130
- DOI: 10.1186/s12879-017-2679-z
Precisão e confiabilidade de um teste imuno-cromatográfico rápido NS1 para diagnóstico DENV-1 no ponto de atendimento e no laboratório
Erratum in
-
Correction to: Accuracy and reliability of an NS1 rapid immunochromatographic test for DENV-1 diagnosis at point of care and in the laboratory.BMC Infect Dis. 2017 Nov 1;17(1):710. doi: 10.1186/s12879-017-2804-z. BMC Infect Dis. 2017. PMID: 29092715 Free PMC article.
Abstract
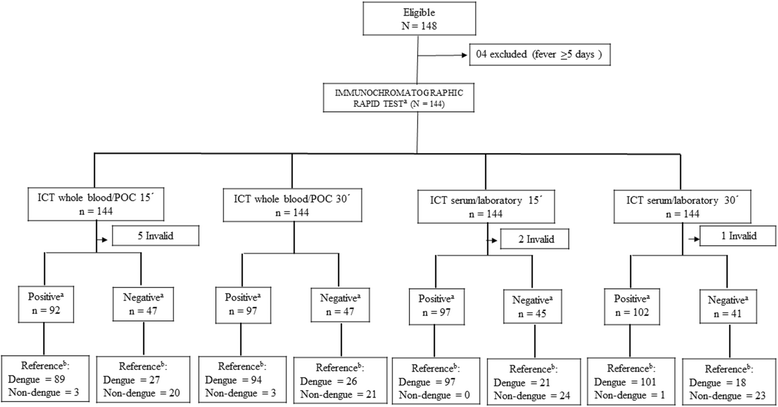
Background: Rapid immunochromatographic tests (ICT) for dengue non-structural protein 1 (NS1) have shown good performance for diagnosing acute-phase dengue in serum in laboratory settings, but rarely have been assessed in whole blood and at point of care (POC). This study compare the accuracy and inter- and intra-observer reliability of the NS1 Bioeasy™ ICT in whole blood at POC versus serum in the laboratory, during a DENV-1 epidemic.
Methods: Cross-sectional study involving 144 adults spontaneously demanding care in an emergency department within 4 days of onset of acute febrile illness. Accuracy of NS1 Bioeasy™ ICT was compared in whole blood and serum, both at 15 and 30 min, blinded to the reference RT-PCR or NS1 ELISA. Non-dengue patients were also tested for Zika virus with RT-PCR. Reliability of whole blood and serum readings by the same or different observers was measured by simple kappa (95% CI).
Results: At 15 min, sensitivity (Sn) of NS1 Bioeasy™ ICT in whole blood/POC was 76.7% (95% CI: 68.0-84.1) and specificity (Sp) was 87.0% (95% CI: 66.4-97.2). Sn in serum/laboratory was 82% (95% CI: 74.1-88.6) and Sp 100% (95% CI: 85.8-100). Positive likelihood ratio was 5.9 (95% CI: 2.0-17.0) for whole blood/POC and 19.8 (95% CI: 2.9-135.1) for serum/laboratory. Reliability of matched readings of whole blood/POC and serum/laboratory by the same observer (k = 0.83, 95% CI: 0.74-0.93) or different observers (k = 0.81, 95% CI: 0.72-0.92) was almost perfect, with higher discordant levels in the absence of dengue. Results did not differ statistically at 5%.
Conclusions: NS1 Bioeasy™ ICT in DENV-1 epidemics is a potentially confirmatory test. Invalid results at 15 min should be reread at 30 min. To optimize impact of implementing ICT in the management of false-negatives it should be incorporated into an algorithm according to setting and available specimen.
Trial registration: UTN U1111-1145-9451 .
Keywords: Dengue; Diagnosis; NS1; Point-of-care systems; Reproducibility of results; Sensitivity and specificity.
Conflict of interest statement
Ethics approval and consent to participate
O estudo foi aprovado pelo Conselho de Revisão Institucional do Instituto de Pesquisa Clínica Evandro Chagas (CAAE 0066.0.009.000-11). Todos os pacientes assinaram o formulário de consentimento livre e esclarecido.
Consentimento para publicação
Não aplicável.
Interesses competitivos
Os autores declaram que não têm interesses concorrentes.
Nota do editor
A Springer Nature permanece neutra em relação a reivindicações jurisdicionais em mapas publicados e afiliações institucionais.
Figures
References
-
- Constenla D, Armien B, Arredondo J, Carabali M, Carrasquilla G, Castro R, et al. Custeio de casos de febre de dengue e surtos: recomendações de um grupo de trabalho de dengue de custo nas Américas. Valorar questões regionais de saúde. 2015;8C:80–91.
-
- Organização Mundial da Saúde. Dengue - diretrizes para diagnóstico, tratamento, prevenção e controle. Genebra: OMS; 2009. p. 11. Disponível em: http://apps.who.int/iris/bitstream/10665/44188/1/9789241547871_eng.pdf. Accessed 9 Apr 2016
-
- Goodman C, Faulkner E, Gould C, Karnes E, Smith A, Aguiar C, et ai. O valor da inovação, adoção e difusão de diagnósticos nos cuidados de saúde. O grupo Lewin, inc. Adv Med. 2005; Disponível em: https://dx.advamed.org/sites/dx.advamed.org/files/resource/Lewin%20Value.... Acessado em 15 de dezembro de 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials