A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial
- PMID: 28851403
- PMCID: PMC5576102
- DOI: 10.1186/s13063-017-2085-2
A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial
Abstract
Background: Pre-operative chemoradiotherapy (CRT) for MRI-defined, locally advanced rectal cancer is primarily intended to reduce local recurrence rates by downstaging tumours, enabling an improved likelihood of curative resection. However, in a subset of patients complete tumour regression occurs implying that no viable tumour is present within the surgical specimen. This raises the possibility that surgery may have been avoided. It is also recognised that response to CRT is a key determinant of prognosis. Recent radiological advances enable this response to be assessed pre-operatively using the MRI tumour regression grade (mrTRG). Potentially, this allows modification of the baseline MRI-derived treatment strategy. Hence, in a 'good' mrTRG responder, with little or no evidence of tumour, surgery may be deferred. Conversely, a 'poor response' identifies an adverse prognostic group which may benefit from additional pre-operative therapy.
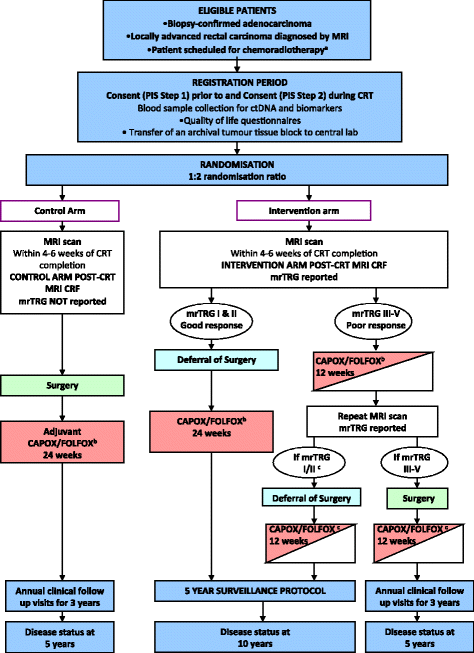
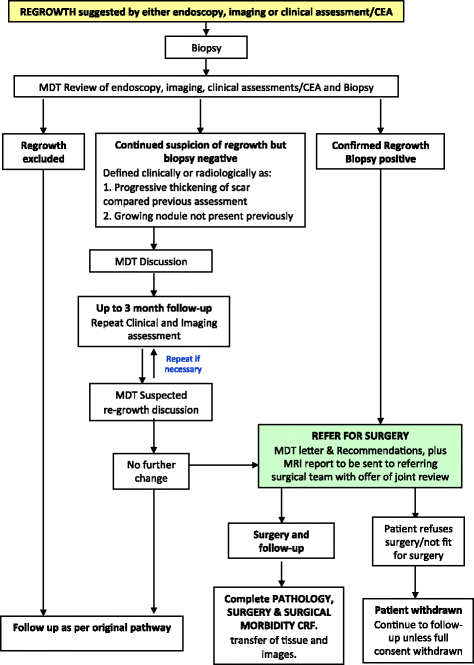
Methods/design: TRIGGER is a multicentre, open, interventional, randomised control feasibility study with an embedded phase III design. Patients with MRI-defined, locally advanced rectal adenocarcinoma deemed to require CRT will be eligible for recruitment. During CRT, patients will be randomised (1:2) between conventional management, according to baseline MRI, versus mrTRG-directed management. The primary endpoint of the feasibility phase is to assess the rate of patient recruitment and randomisation. Secondary endpoints include the rate of unit recruitment, acute drug toxicity, reproducibility of mrTRG reporting, surgical morbidity, pathological circumferential resection margin involvement, pathology regression grade, residual tumour cell density and surgical/specimen quality rates. The phase III trial will focus on long-term safety, regrowth rates, oncological survival analysis, quality of life and health economics analysis.
Discussion: The TRIGGER trial aims to determine whether patients with locally advanced rectal cancer can be recruited and subsequently randomised into a control trial that offers MRI-directed patient management according to radiological response to CRT (mrTRG). The feasibility study will inform a phase III trial design investigating stratified treatment of good and poor responders according to 3-year disease-free survival, colostomy-free survival as well as an increase in cases managed without a major resection.
Trial registration: ClinicalTrials.gov, ID: NCT02704520 . Registered on 5 February 2016.
Keywords: Chemoradiotherapy; Complete response; Randomised control trial; Rectal cancer; Tumour cell density; Tumour regression; mrTRG.
Conflict of interest statement
Ethics approval and consent to participate
National Research Ethics Committee approval was granted by London – Surrey Borders Research Ethics Committee (REC) on 18 December 2015 (15/LO/1836). The REC issued centralised ethics committee approval in all UK centres. The conduct of the trial has been, and will continue to be, in accordance with the clinical trials regulations, the principles of Good Clinical Practice and the Declaration of Helsinki. A prerequisite to recruiting each patient to trial has been, and will continue to be, signed informed consent to participate.
Version 5.0 of the full trial protocol, including the appendices for the delivery and assessment of all applicable imaging techniques, chemotherapy, radiotherapy, and pathology are available as supporting documents; however, publication of supporting raw data is currently not applicable.
Consent for publication
Informed written consent was received for publication of the manuscript and figures. Written informed consent was obtained from the patients to participate and for publication of their individual details and accompanying anonymised images in this manuscript. The consent form is held by the Royal Marsden Trials Unit and is available for review by the Editor-in-Chief.
Competing interests
Professor David Cunningham receives research income from Amgen, AstraZeneca, Bayer, Celgene, Merrimack, Medimmune, Merck Serono and Sanofi. Prof Phil Quirke has pharmaceutical research contracts with Roche/Ventana and Eisai, and is a member of an advisory board with AMGEN. All other authors have no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

