REstricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH): study protocol for a pilot randomised controlled trial
- PMID: 28851407
- PMCID: PMC5576288
- DOI: 10.1186/s13063-017-2137-7
REstricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH): study protocol for a pilot randomised controlled trial
Abstract
Background: Guidelines recommend an initial intravenous (IV) fluid bolus of 30 ml/kg isotonic crystalloid for patients with sepsis and hypotension. However, there is a lack of evidence from clinical trials to support this. Accumulating observational data suggest harm associated with the injudicious use of fluids in sepsis. There is currently equipoise regarding liberal or restricted fluid-volume resuscitation as first-line treatment for sepsis-related hypotension. A randomised trial comparing these two approaches is, therefore, justified.
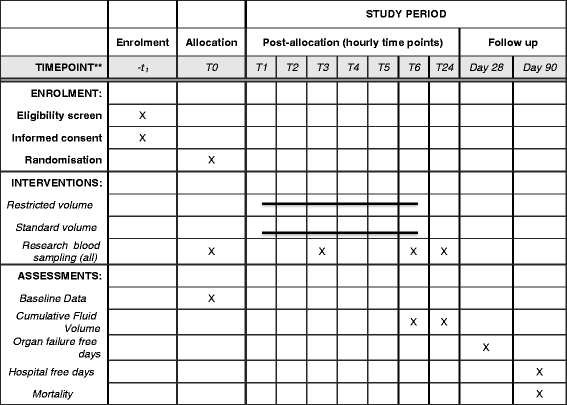
Methods/design: The REstricted Fluid REsuscitation in Sepsis-associated Hypotension trial (REFRESH) is a multicentre, open-label, randomised, phase II clinical feasibility trial. Participants will be patients presenting to the emergency departments of Australian metropolitan hospitals with suspected sepsis and a systolic blood pressure of < 100 mmHg, persisting after a 1000-ml fluid bolus with isotonic crystalloid. Participants will be randomised to either a second 1000-ml fluid bolus (standard care) or maintenance rate fluid only, with the early commencement of a vasopressor infusion to maintain a mean arterial pressure of > 65 mmHg, if required (restricted fluid). All will receive further protocolised fluid boluses (500 ml or 250 ml, respectively), if required during the 6-h study period. The primary outcome measure is total volume administered in the first 6 h. Secondary outcomes include fluid volume at 24 h, organ support 'free days' to day 28, 90-day mortality, and a range of feasibility and process-of-care measures. Participants will also undergo serial measurement, over the first 24 h, of biomarkers of inflammation, endothelial cell activation and glycocalyx degradation for comparison between the groups.
Discussion: This is the first randomised trial examining fluid volume for initial resuscitation in septic shock in an industrialised country. A pragmatic, open-label design will establish the feasibility of undertaking a large, international, multicentre trial with sufficient power to assess clinical outcomes. The embedded biomarker study aims to provide mechanistic plausibility for a larger trial by defining the effects of fluid volume on markers of systemic inflammation and the vascular endothelium.
Trial registration: Australia and New Zealand Clinical Trials Registry, ID: ACTRN12616000006448. Registered on 12 January 2016.
Keywords: Fluid therapy; Hypotension; Sepsis.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
The trial protocol has been endorsed by the Human Research Ethics Committees:
South Metropolitan Health Service, Western Australia (Reference 15-114, 13 October 2015) for Western Australian Sites
Austin Health, Victoria (HREC/15/Austin/486, 20 April 2016) for all other sites
The Australian National Health and Medical Research Council (NHMRC) National Statement on the Ethical Conduct of Research in Humans, Chapter 4.4 addresses the conduct of research among people highly dependent upon medical care and who may be unable to give consent [35]. This includes emergency and intensive care settings, such as applies in the present study, where research is necessary to improve the safety and efficiency of interventions used in their treatment.
Participants in this trial will have low blood pressure in the setting of sepsis and there is a short time window in which this must be treated by the administration of one of the two trial interventions. Several approaches will be adopted. When a conscious and comprehending patient is eligible, a brief verbal explanation of the study (following a standard script) will be given and a provisional verbal consent obtained to allow randomisation and initiation of timely intervention. This script is attached as an Appendix (see Additional file 5). This will be followed by provision of a written Participant Information Sheet. If the patient chooses not to continue they will be withdrawn from the trial and their care will revert to standard management according to the treating physician. This will be recorded in the trial screening logs. Where a patient’s condition precludes giving prospective informed consent, they may be enrolled under an initial waiver of consent (or procedural authorisation in Victoria). Following this, formal consent will be obtained from the ‘person responsible’ for the patient, generally a close family member. This may be in person, or by telephone. If telephone consent is obtained this will be clearly documented in the clinical notes, and followed up by a formal written consent, as soon as practicable.
When the participant regains capacity, consent will be sought to continue in the study. Participants may choose to withdraw from the study (or be withdrawn by the ‘person responsible’ if enrolled under waiver of consent) at any stage. Under these circumstances, permission will be sought to use data and samples collected to that time point. This withdrawal will be recorded in the study logs and care will revert to standard management according to the treating team. The consent process is summarised in (see Additional file 6: Figure S4).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): a pilot randomised controlled trial.Intensive Care Med. 2018 Dec;44(12):2070-2078. doi: 10.1007/s00134-018-5433-0. Epub 2018 Oct 31. Intensive Care Med. 2018. PMID: 30382308 Clinical Trial.
-
A trial to determine whether septic shock-reversal is quicker in pediatric patients randomized to an early goal-directed fluid-sparing strategy versus usual care (SQUEEZE): study protocol for a pilot randomized controlled trial.Trials. 2016 Nov 22;17(1):556. doi: 10.1186/s13063-016-1689-2. Trials. 2016. PMID: 27876084 Free PMC article. Clinical Trial.
-
Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial.JAMA. 2017 Oct 3;318(13):1233-1240. doi: 10.1001/jama.2017.10913. JAMA. 2017. PMID: 28973227 Free PMC article. Clinical Trial.
-
Balanced crystalloids for septic shock resuscitation.Rev Bras Ter Intensiva. 2016 Oct-Dec;28(4):463-471. doi: 10.5935/0103-507X.20160079. Rev Bras Ter Intensiva. 2016. PMID: 28099643 Free PMC article. Review.
-
Liberal Versus Restrictive Intravenous Fluid Therapy for Early Septic Shock: Rationale for a Randomized Trial.Ann Emerg Med. 2018 Oct;72(4):457-466. doi: 10.1016/j.annemergmed.2018.03.039. Epub 2018 May 10. Ann Emerg Med. 2018. PMID: 29753517 Free PMC article.
Cited by
-
Association of Positive Fluid Balance at Discharge After Sepsis Management With 30-Day Readmission.JAMA Netw Open. 2021 Jun 1;4(6):e216105. doi: 10.1001/jamanetworkopen.2021.6105. JAMA Netw Open. 2021. PMID: 34086036 Free PMC article.
-
Pragmatic Pediatric Trial of Balanced Versus Normal Saline Fluid in Sepsis: The PRoMPT BOLUS Randomized Controlled Trial Pilot Feasibility Study.Acad Emerg Med. 2019 Dec;26(12):1346-1356. doi: 10.1111/acem.13815. Epub 2019 Jul 18. Acad Emerg Med. 2019. PMID: 31183919 Free PMC article.
-
Fluid Response Evaluation in Sepsis Hypotension and Shock: A Randomized Clinical Trial.Chest. 2020 Oct;158(4):1431-1445. doi: 10.1016/j.chest.2020.04.025. Epub 2020 Apr 27. Chest. 2020. PMID: 32353418 Free PMC article. Clinical Trial.
-
The Effects of Resuscitative Fluid Therapy on the Endothelial Surface Layer.Front Vet Sci. 2021 May 7;8:661660. doi: 10.3389/fvets.2021.661660. eCollection 2021. Front Vet Sci. 2021. PMID: 34026896 Free PMC article. Review.
-
Prognostic value of hemodynamic indices in patients with sepsis after fluid resuscitation.World J Clin Cases. 2021 May 6;9(13):3008-3013. doi: 10.12998/wjcc.v9.i13.3008. World J Clin Cases. 2021. PMID: 33969086 Free PMC article.
References
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. Surviving Sepsis Campaign: international guidelines for the management of severe sepsis and septic shock 2016. Crit Care Med. 2017. doi:10.1097/CCM.0000000000002255. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical