The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting
- PMID: 28851423
- PMCID: PMC5574249
- DOI: 10.1186/s13058-017-0861-2
The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting
Abstract
Background: Patients with BRCA1-like tumors correlate with improved response to DNA double-strand break-inducing therapy. A gene expression-based classifier was developed to distinguish between BRCA1-like and non-BRCA1-like tumors. We hypothesized that these tumors may also be more sensitive to PARP inhibitors than standard treatments.
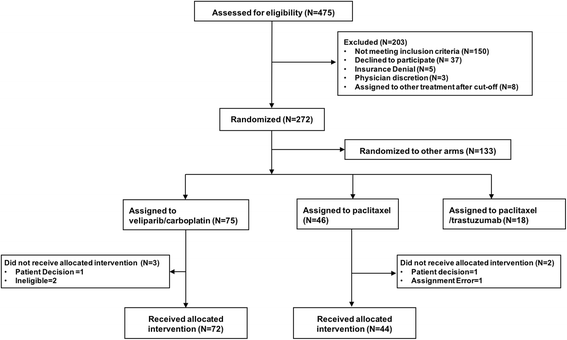
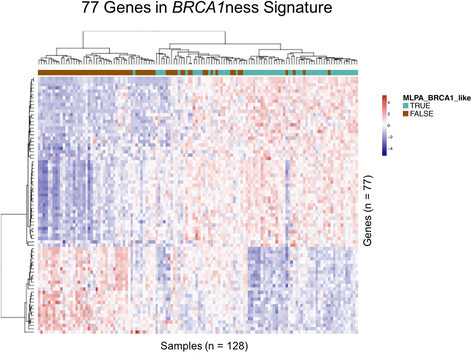
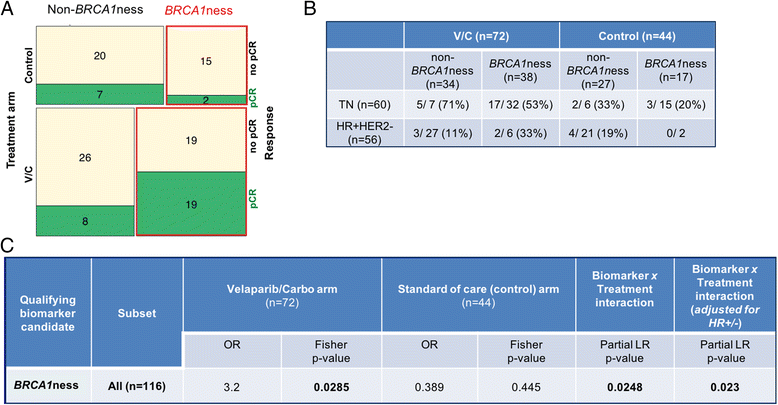
Methods: A diagnostic gene expression signature (BRCA1ness) was developed using a centroid model with 128 triple-negative breast cancer samples from the EU FP7 RATHER project. This BRCA1ness signature was then tested in HER2-negative patients (n = 116) from the I-SPY 2 TRIAL who received an oral PARP inhibitor veliparib in combination with carboplatin (V-C), or standard chemotherapy alone. We assessed the association between BRCA1ness and pathologic complete response in the V-C and control arms alone using Fisher's exact test, and the relative performance between arms (biomarker × treatment interaction, likelihood ratio p < 0.05) using a logistic model and adjusting for hormone receptor status (HR).
Results: We developed a gene expression signature to identify BRCA1-like status. In the I-SPY 2 neoadjuvant setting the BRCA1ness signature associated significantly with response to V-C (p = 0.03), but not in the control arm (p = 0.45). We identified a significant interaction between BRCA1ness and V-C (p = 0.023) after correcting for HR.
Conclusions: A genomic-based BRCA1-like signature was successfully translated to an expression-based signature (BRC1Aness). In the I-SPY 2 neoadjuvant setting, we determined that the BRCA1ness signature is capable of predicting benefit of V-C added to standard chemotherapy compared to standard chemotherapy alone.
Trial registration: I-SPY 2 TRIAL beginning December 31, 2009: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer (I-SPY 2), NCT01042379 .
Keywords: BRCAness; Breast cancer; Neoadjuvant; PARP inhibition; Triple-negative breast cancer.
Conflict of interest statement
Ethics approval and consent to participate
All patients for the I-SPY 2 clinical trial provided written informed consent. All participating sites received institutional review board approval (Additional file 2: Table S2).
Competing interests
SL, TMS, IMS and JP are all named coinventors on a patent application for a
Consent for publication
All authors consent to publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Linn SC, van ‘t Veer LJ. Clinical relevance of the triple-negative breast cancer concept: genetic basis and clinical utility of the concept. Eur J Cancer Oxf Engl 1990. 2009;45(Suppl 1):11–26. - PubMed
-
- Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

