Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia
- PMID: 28851445
- PMCID: PMC5576380
- DOI: 10.1186/s13045-017-0519-7
Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia
Abstract
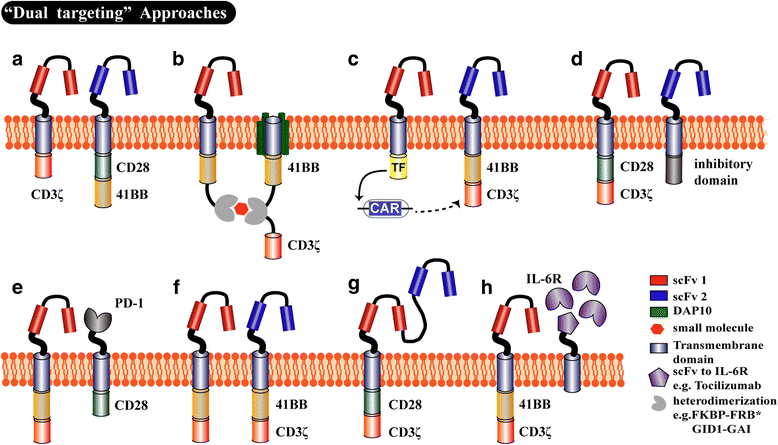
Currently, conventional therapies for acute myeloid leukemia (AML) have high failure and relapse rates. Thus, developing new strategies is crucial for improving the treatment of AML. With the clinical success of anti-CD19 chimeric antigen receptor (CAR) T cell therapies against B-lineage malignancies, many studies have attempted to translate the success of CAR T cell therapy to other malignancies, including AML. This review summarizes the current advances in CAR T cell therapy against AML, including preclinical studies and clinical trials, and discusses the potential AML-associated surface markers that could be used for further CAR technology. Finally, we describe strategies that might address the current issues of employing CAR T cell therapy in AML.
Keywords: Acute myeloid leukemia; Chimeric antigen receptors; Immunotherapy.
Conflict of interest statement
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
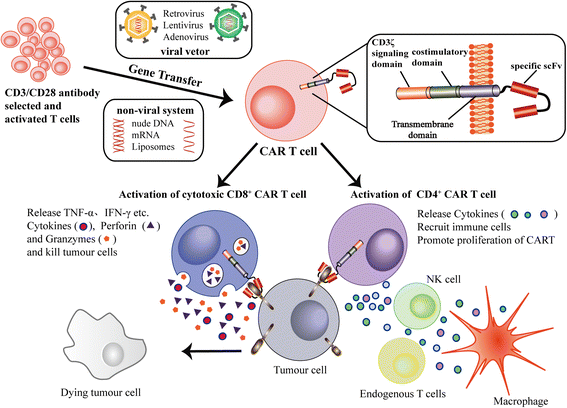
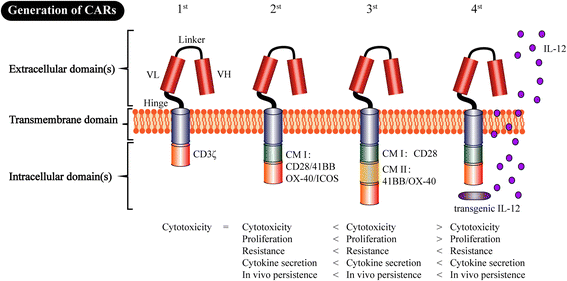
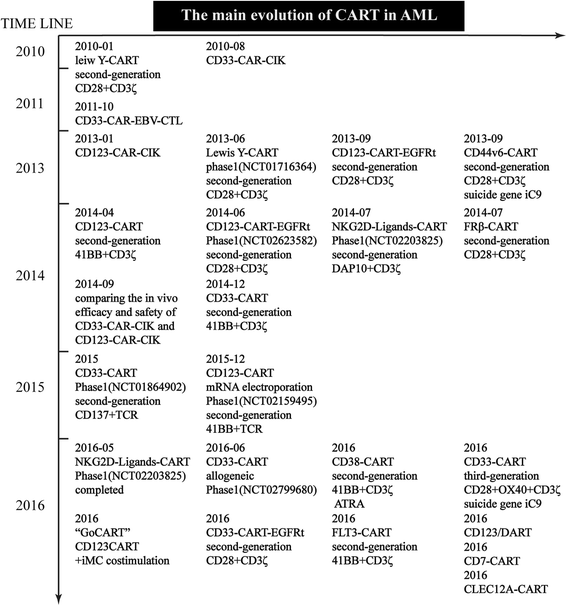
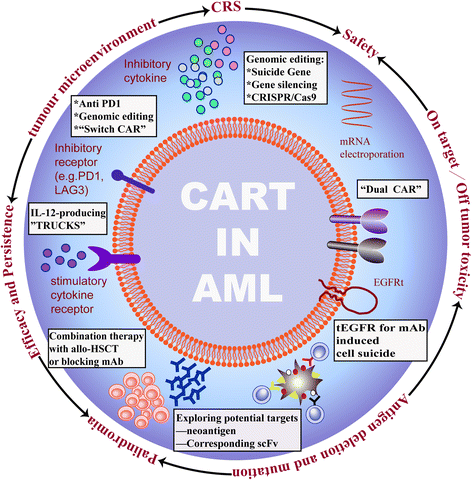
References
-
- Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454–462. - PubMed
-
- Yates JW, Wallace J, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–488. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

