The complexity of TRIM28 contribution to cancer
- PMID: 28851455
- PMCID: PMC5574234
- DOI: 10.1186/s12929-017-0374-4
The complexity of TRIM28 contribution to cancer
Abstract
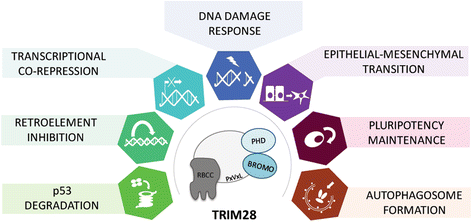
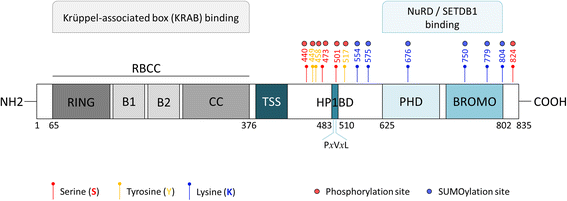
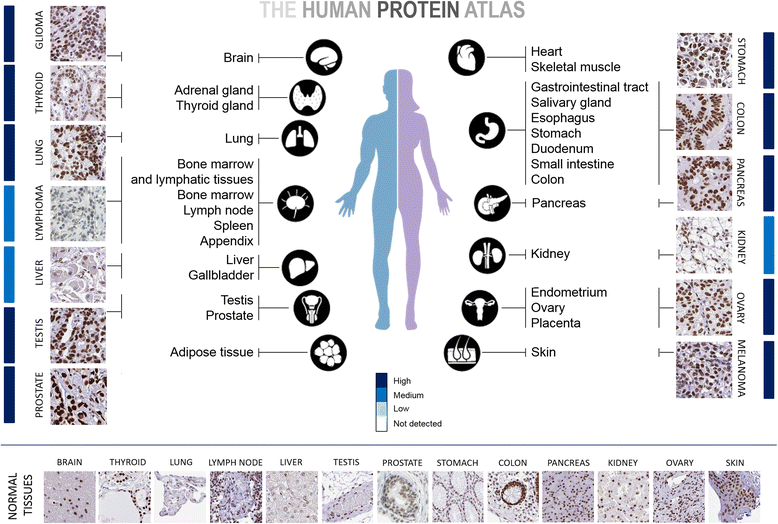
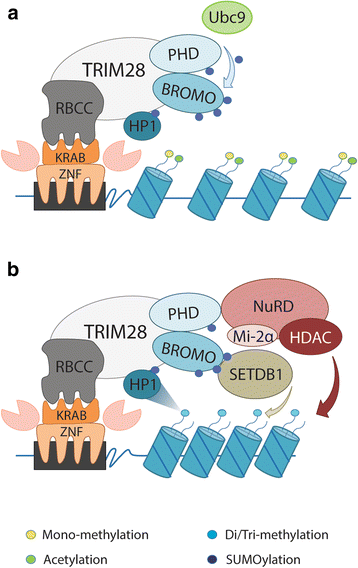
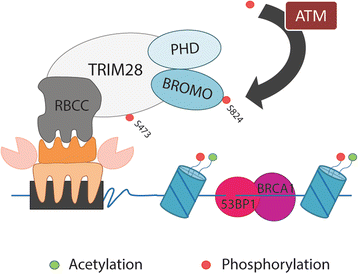
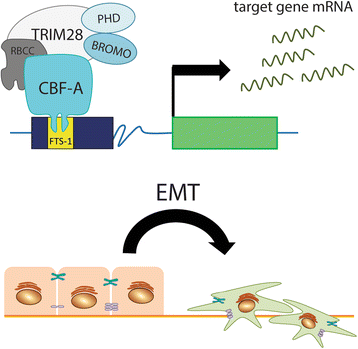
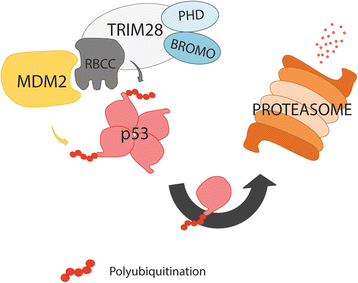
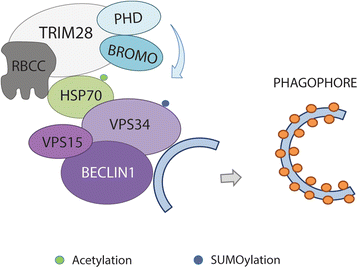
Since the first discovery in 1996, the engagement of TRIM28 in distinct aspects of cellular biology has been extensively studied resulting in identification of a complex nature of TRIM28 protein. In this review, we summarize core biological functions of TRIM28 that emerge from TRIM28 multi-domain structure and possessed enzymatic activities. Moreover, we will discuss whether the complexity of TRIM28 engagement in cancer biology makes TRIM28 a possible candidate for targeted anti-cancer therapy. Briefly, we will demonstrate the role of TRIM28 in regulation of target gene transcription, response to DNA damage, downregulation of p53 activity, stimulation of epithelial-to-mesenchymal transition, stemness sustainability, induction of autophagy and regulation of retrotransposition, to provide the answer whether TRIM28 functions as a stimulator or inhibitor of tumorigenesis. To date, number of studies demonstrate significant upregulation of TRIM28 expression in cancer tissues which correlates with worse overall patient survival, suggesting that TRIM28 supports cancer progression. Here, we present distinct aspects of TRIM28 involvement in regulation of cancer cell homeostasis which collectively imply pro-tumorigenic character of TRIM28. Thorough analyses are further needed to verify whether TRIM28 possess the potential to become a new anti-cancer target.
Keywords: Autophagy; Cancer; Cancer stem cells; EMT; KAP1; TRIM28; Transcriptional co-repressor.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

