Effects of a brief, pedometer-based behavioral intervention for individuals with COPD during inpatient pulmonary rehabilitation on 6-week and 6-month objectively measured physical activity: study protocol for a randomized controlled trial
- PMID: 28851462
- PMCID: PMC5576331
- DOI: 10.1186/s13063-017-2124-z
Effects of a brief, pedometer-based behavioral intervention for individuals with COPD during inpatient pulmonary rehabilitation on 6-week and 6-month objectively measured physical activity: study protocol for a randomized controlled trial
Abstract
Background: Pulmonary rehabilitation programs often fail to substantially enhance long-term physical activity in patients with chronic obstructive pulmonary disease (COPD). The reasons for successful physical activity changes in patients with COPD are not well understood. The need to better understand the determinants of physical activity in patients with COPD and effective rehabilitation strategies to improve physical activity is evident.
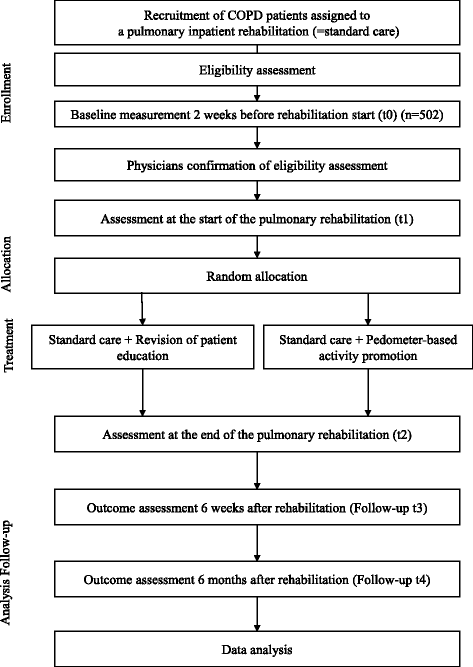
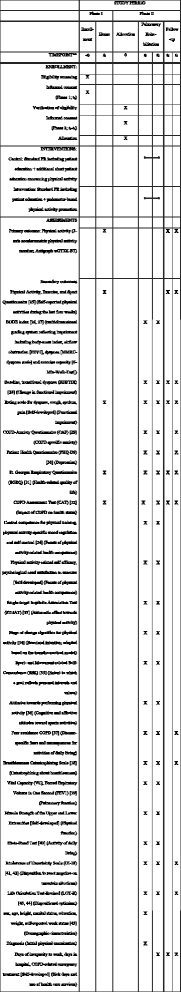
Methods/design: The STAR study (Stay Active after Rehabilitation) investigates, in a randomized controlled trial, the additional effect of a pedometer-based behavior-change intervention during inpatient pulmonary rehabilitation on objectively measured physical activity 6 weeks and 6 months post rehabilitation. The intervention uses the behavior-change techniques (1) instruction on how, where and when to perform the behavior, (2) prompt goal setting for physical activity, (3) prompt self-monitoring of behavior, and (4) feedback on behavior. The primary outcome of physical activity will be measured using a physical activity monitor (Actigraph wGT3X-BT) for a period of 7 days, firstly 2 weeks before rehabilitation begins (t0) as well as 6 weeks and 6 months after rehabilitation (t3, t4). Additionally, to predict physical activity progression after rehabilitation, a complex personal diagnostics battery, including questionnaires as well as functional assessments, is to be carried out at the start and end of rehabilitation (t1, t2). This battery is based on the foundational ideas of the Physical Activity-Related health Competence model. Five hundred and two patients with COPD, aged 18 years or older and admitted for an approved pulmonary rehabilitation, will be enrolled in the STAR study.
Discussion: The STAR study is designed as a randomized controlled trial to gain a better understanding of the personal determinants of physical activity in patients with COPD and to evaluate a pedometer-based physical activity-change intervention in the context of inpatient pulmonary rehabilitation. The results enable the future identification of patients with COPD who will find it difficult to engage in long-term physical activity after rehabilitation. Based on this, intervention strategies to promote physical activity in the content of pulmonary rehabilitation can be optimized.
Trial registration: Clinicaltrials.gov, ID: NCT02966561 . Registered retrospectively after the start of the recruitment in June 2016 on 22 November 2016. All protocol modifications will be registered in the trial registry.
Keywords: Accelerometry; Behavior-change intervention; Chronic obstructive pulmonary disease (COPD); Health-related quality of life (HRQoL); Implicit association test; Pedometer; Physical activity-related health competence.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the independent Research Ethics Committee of the Medical Faculty of Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany in 2015 (Ref. No. 321_15B).
The Department of Data Privacy Collection of the German Pension Insurance Association, Section Bavaria South approved the STAR study data security model to ensure confidentiality before, during, and after the trial. Signed informed consent of the participants is a prerequisite for taking part in the STAR study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical