Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer
- PMID: 28851690
- PMCID: PMC5626664
- DOI: 10.1158/1940-6207.CAPR-17-0198
Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer
Retraction in
-
Retraction: Tobacco-specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer.Cancer Prev Res (Phila). 2024 Jun 4;17(6):281. doi: 10.1158/1940-6207.CAPR-24-0164. Cancer Prev Res (Phila). 2024. PMID: 38831722 No abstract available.
Expression of concern in
-
Expression of Concern: Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer.Cancer Prev Res (Phila). 2019 Oct;12(10):733-734. doi: 10.1158/1940-6207.CAPR-19-0406. Epub 2019 Sep 3. Cancer Prev Res (Phila). 2019. PMID: 31481538 No abstract available.
Abstract
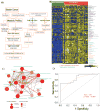
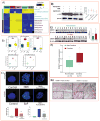
Smoking is a major risk factor for the development of bladder cancer; however, the functional consequences of the carcinogens in tobacco smoke and bladder cancer-associated metabolic alterations remain poorly defined. We assessed the metabolic profiles in bladder cancer smokers and non-smokers and identified the key alterations in their metabolism. LC/MS and bioinformatic analysis were performed to determine the metabolome associated with bladder cancer smokers and were further validated in cell line models. Smokers with bladder cancer were found to have elevated levels of methylated metabolites, polycyclic aromatic hydrocarbons, DNA adducts, and DNA damage. DNA methyltransferase 1 (DNMT1) expression was significantly higher in smokers than non-smokers with bladder cancer. An integromics approach, using multiple patient cohorts, revealed strong associations between smokers and high-grade bladder cancer. In vitro exposure to the tobacco smoke carcinogens, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene (BaP) led to increase in levels of methylated metabolites, DNA adducts, and extensive DNA damage in bladder cancer cells. Cotreatment of bladder cancer cells with these carcinogens and the methylation inhibitor 5-aza-2'-deoxycytidine rewired the methylated metabolites, DNA adducts, and DNA damage. These findings were confirmed through the isotopic-labeled metabolic flux analysis. Screens using smoke-associated metabolites and DNA adducts could provide robust biomarkers and improve individual risk prediction in bladder cancer smokers. Noninvasive predictive biomarkers that can stratify the risk of developing bladder cancer in smokers could aid in early detection and treatment. Cancer Prev Res; 10(10); 588-97. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
High Level of Tobacco Carcinogen-Derived DNA Damage in Oral Cells Is an Independent Predictor of Oral/Head and Neck Cancer Risk in Smokers.Cancer Prev Res (Phila). 2017 Sep;10(9):507-513. doi: 10.1158/1940-6207.CAPR-17-0140. Epub 2017 Jul 5. Cancer Prev Res (Phila). 2017. PMID: 28679497 Free PMC article.
-
Detection of DNA adducts derived from the tobacco carcinogens, benzo[a]pyrene and dibenzo[def,p]chrysene in human oral buccal cells.Carcinogenesis. 2022 Sep 19;43(8):746-753. doi: 10.1093/carcin/bgac058. Carcinogenesis. 2022. PMID: 35749296 Free PMC article.
-
Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):E6152-E6161. doi: 10.1073/pnas.1804869115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915082 Free PMC article.
-
DNA adducts in human tissues: biomarkers of exposure to carcinogens in tobacco smoke.Environ Health Perspect. 1996 May;104 Suppl 3(Suppl 3):453-8. doi: 10.1289/ehp.96104s3453. Environ Health Perspect. 1996. PMID: 8781363 Free PMC article. Review.
-
DNA adduct formation from tobacco-specific N-nitrosamines.Mutat Res. 1999 Mar 8;424(1-2):127-42. doi: 10.1016/s0027-5107(99)00014-7. Mutat Res. 1999. PMID: 10064856 Review.
Cited by
-
Facets of Communication: Gap Junction Ultrastructure and Function in Cancer Stem Cells and Tumor Cells.Cancers (Basel). 2019 Mar 1;11(3):288. doi: 10.3390/cancers11030288. Cancers (Basel). 2019. PMID: 30823688 Free PMC article. Review.
-
An Integrated Genomic Strategy to Identify CHRNB4 as a Diagnostic/Prognostic Biomarker for Targeted Therapy in Head and Neck Cancer.Cancers (Basel). 2020 May 22;12(5):1324. doi: 10.3390/cancers12051324. Cancers (Basel). 2020. PMID: 32455963 Free PMC article.
-
History of the Relationship Between Smoking and Bladder Cancer: A Public Health Perspective.Urology. 2023 Jan;171:6-10. doi: 10.1016/j.urology.2022.08.007. Epub 2022 Aug 14. Urology. 2023. PMID: 35977631 Free PMC article. No abstract available.
-
Mouse Models for Immune Checkpoint Blockade Therapeutic Research in Oral Cancer.Int J Mol Sci. 2022 Aug 16;23(16):9195. doi: 10.3390/ijms23169195. Int J Mol Sci. 2022. PMID: 36012461 Free PMC article. Review.
-
Measurement of methylated metabolites using Liquid Chromatography-Mass Spectrometry and its biological application.Anal Methods. 2019 Jan 7;11(1):49-57. doi: 10.1039/C8AY02168F. Epub 2018 Nov 15. Anal Methods. 2019. PMID: 31762797 Free PMC article.
References
-
- Smart CR. Bladder cancer survival statistics. J Occup Med. 1990;32(9):926–8. - PubMed
-
- Rink M, et al. Influence of preoperatively detected circulating tumor cells on the outcome of patients with urothelial carcinoma of the bladder treated with radical cystectomy. J Clin Oncol. 2012;30(5_suppl):268.
-
- Nuhn P, et al. Prognostic value of prior history of urothelial carcinoma of the bladder in patients with upper urinary tract urothelial carcinoma: results from a retrospective multicenter study. World J Urol. 2015;33(7):1005–13. - PubMed
-
- Scosyrev E, et al. The burden of bladder cancer in men and women: analysis of the years of life lost. BJU Int. 2012;109(1):57–62. - PubMed
-
- Brennan P, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86(2):289–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 CA145444/CA/NCI NIH HHS/United States
- T32 HD007495/HD/NICHD NIH HHS/United States
- F30 CA196108/CA/NCI NIH HHS/United States
- R01 CA133458/CA/NCI NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- P50 CA186786/CA/NCI NIH HHS/United States
- R21 CA205257/CA/NCI NIH HHS/United States
- R21 CA179720/CA/NCI NIH HHS/United States
- R21 CA173150/CA/NCI NIH HHS/United States
- U01 CA167234/CA/NCI NIH HHS/United States
- R01 CA220297/CA/NCI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01 CA184208/CA/NCI NIH HHS/United States
- P30 HD007495/HD/NICHD NIH HHS/United States
- U01 CA111275/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

