Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development
- PMID: 28851704
- PMCID: PMC5665481
- DOI: 10.1242/dev.151142
Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development
Abstract
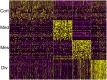
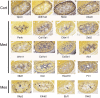
Single-cell RNA-seq is a powerful technique. Nevertheless, there are important limitations, including the technical challenges of breaking down an organ or tissue into a single-cell suspension. Invariably, this has required enzymatic incubation at 37°C, which can be expected to result in artifactual changes in gene expression patterns. Here, we describe a dissociation method that uses a protease with high activity in the cold, purified from a psychrophilic microorganism. The entire procedure is carried out at 6°C or colder, at which temperature mammalian transcriptional machinery is largely inactive, thereby effectively 'freezing in' the in vivo gene expression patterns. To test this method, we carried out RNA-seq on 20,424 single cells from postnatal day 1 mouse kidneys, comparing the results of the psychrophilic protease method with procedures using 37°C incubation. We show that the cold protease method provides a great reduction in gene expression artifacts. In addition, the results produce a single-cell resolution gene expression atlas of the newborn mouse kidney, an interesting time in development when mature nephrons are present yet nephrogenesis remains extremely active.
Keywords: Artifacts; Cell dissociation; Kidney development; RNA-seq; Single cell.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

