Gpr158 mediates osteocalcin's regulation of cognition
- PMID: 28851741
- PMCID: PMC5626410
- DOI: 10.1084/jem.20171320
Gpr158 mediates osteocalcin's regulation of cognition
Abstract
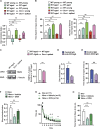
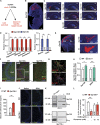
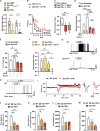
That osteocalcin (OCN) is necessary for hippocampal-dependent memory and to prevent anxiety-like behaviors raises novel questions. One question is to determine whether OCN is also sufficient to improve these behaviors in wild-type mice, when circulating levels of OCN decline as they do with age. Here we show that the presence of OCN is necessary for the beneficial influence of plasma from young mice when injected into older mice on memory and that peripheral delivery of OCN is sufficient to improve memory and decrease anxiety-like behaviors in 16-mo-old mice. A second question is to identify a receptor transducing OCN signal in neurons. Genetic, electrophysiological, molecular, and behavioral assays identify Gpr158, an orphan G protein-coupled receptor expressed in neurons of the CA3 region of the hippocampus, as transducing OCN's regulation of hippocampal-dependent memory in part through inositol 1,4,5-trisphosphate and brain-derived neurotrophic factor. These results indicate that exogenous OCN can improve hippocampal-dependent memory in mice and identify molecular tools to harness this pathway for therapeutic purposes.
© 2017 Khrimian et al.
Figures

References
-
- David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.P., et al. . 2009. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 62:479–493. 10.1016/j.neuron.2009.04.017 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

