Activation of master virulence regulator PhoP in acidic pH requires the Salmonella-specific protein UgtL
- PMID: 28851823
- PMCID: PMC5966036
- DOI: 10.1126/scisignal.aan6284
Activation of master virulence regulator PhoP in acidic pH requires the Salmonella-specific protein UgtL
Abstract
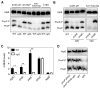
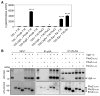
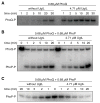
Acidic conditions, such as those inside phagosomes, stimulate the intracellular pathogen Salmonella enterica to activate virulence genes. The sensor PhoQ responds to a mildly acidic pH by phosphorylating, and thereby activating, the virulence regulator PhoP. This PhoP/PhoQ two-component system is conserved in a subset of Gram-negative bacteria. PhoQ is thought to be sufficient to activate PhoP in mildly acidic pH. However, we found that the Salmonella-specific protein UgtL, which was horizontally acquired by Salmonella before the divergence of S. enterica and Salmonella bongori, was also necessary for PhoQ to activate PhoP under mildly acidic pH conditions but not for PhoQ to activate PhoP in response to low Mg2+ or the antimicrobial peptide C18G. UgtL increased the abundance of phosphorylated PhoP by stimulating autophosphorylation of PhoQ, thereby increasing the amount of the phosphodonor for PhoP. Deletion of ugtL attenuated Salmonella virulence and further reduced PhoP activation in a strain bearing a form of PhoQ that is not responsive to acidic pH. These data suggest that when Salmonella experiences mildly acidic pH, PhoP activation requires PhoQ to detect pH and UgtL to amplify the PhoQ response. Our findings reveal how acquisition of a foreign gene can strengthen signal responsiveness in an ancestral regulatory system.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

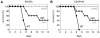
Similar articles
-
Horizontally acquired regulatory gene activates ancestral regulatory system to promote Salmonella virulence.Nucleic Acids Res. 2020 Nov 4;48(19):10832-10847. doi: 10.1093/nar/gkaa813. Nucleic Acids Res. 2020. PMID: 33045730 Free PMC article.
-
Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence.Mol Microbiol. 2016 Sep;101(6):1024-38. doi: 10.1111/mmi.13439. Epub 2016 Jul 8. Mol Microbiol. 2016. PMID: 27282333 Free PMC article.
-
Genome expression analysis of nonproliferating intracellular Salmonella enterica serovar Typhimurium unravels an acid pH-dependent PhoP-PhoQ response essential for dormancy.Infect Immun. 2013 Jan;81(1):154-65. doi: 10.1128/IAI.01080-12. Epub 2012 Oct 22. Infect Immun. 2013. PMID: 23090959 Free PMC article.
-
The PhoQ/PhoP regulatory network of Salmonella enterica.Adv Exp Med Biol. 2008;631:7-21. doi: 10.1007/978-0-387-78885-2_2. Adv Exp Med Biol. 2008. PMID: 18792679 Review.
-
How the PhoP/PhoQ System Controls Virulence and Mg2+ Homeostasis: Lessons in Signal Transduction, Pathogenesis, Physiology, and Evolution.Microbiol Mol Biol Rev. 2021 Aug 18;85(3):e0017620. doi: 10.1128/MMBR.00176-20. Epub 2021 Jun 30. Microbiol Mol Biol Rev. 2021. PMID: 34191587 Free PMC article. Review.
Cited by
-
Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases.Biomolecules. 2021 Oct 15;11(10):1524. doi: 10.3390/biom11101524. Biomolecules. 2021. PMID: 34680156 Free PMC article. Review.
-
Conserved patterns of sequence diversification provide insight into the evolution of two-component systems in Enterobacteriaceae.Microb Genom. 2024 Mar;10(3):001215. doi: 10.1099/mgen.0.001215. Microb Genom. 2024. PMID: 38502064 Free PMC article.
-
A novel type of colistin resistance genes selected from random sequence space.PLoS Genet. 2021 Jan 7;17(1):e1009227. doi: 10.1371/journal.pgen.1009227. eCollection 2021 Jan. PLoS Genet. 2021. PMID: 33411736 Free PMC article.
-
Large Roles of Small Proteins.Annu Rev Microbiol. 2024 Nov;78(1):1-22. doi: 10.1146/annurev-micro-112723-083001. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 38772630 Free PMC article. Review.
-
Impacts of Hydrophobic Mismatch on Antimicrobial Peptide Efficacy and Bilayer Permeabilization.Antibiotics (Basel). 2023 Nov 14;12(11):1624. doi: 10.3390/antibiotics12111624. Antibiotics (Basel). 2023. PMID: 37998826 Free PMC article.
References
-
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. - PubMed
-
- Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl--channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 2000;408:369–373. - PubMed
-
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. - PubMed
-
- Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–331. - PubMed
-
- Schneider D, Gerhardt E, Bock J, Muller MM, Wolburg H, Lang F, Schulz JB. Intracellular acidification by inhibition of the Na+/H+-exchanger leads to caspase-independent death of cerebellar granule neurons resembling paraptosis. Cell Death Differ. 2004;11:760–770. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

