The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing
- PMID: 28851844
- PMCID: PMC5574709
- DOI: 10.1128/mBio.00812-17
The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing
Abstract
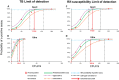
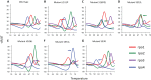
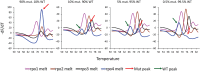
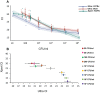
The Xpert MTB/RIF assay (Xpert) is a rapid test for tuberculosis (TB) and rifampin resistance (RIF-R) suitable for point-of-care testing. However, it has decreased sensitivity in smear-negative sputum, and false identification of RIF-R occasionally occurs. We developed the Xpert MTB/RIF Ultra assay (Ultra) to improve performance. Ultra and Xpert limits of detection (LOD), dynamic ranges, and RIF-R rpoB mutation detection were tested on Mycobacterium tuberculosis DNA or sputum samples spiked with known numbers of M. tuberculosis H37Rv or Mycobacterium bovis BCG CFU. Frozen and prospectively collected clinical samples from patients suspected of having TB, with and without culture-confirmed TB, were also tested. For M. tuberculosis H37Rv, the LOD was 15.6 CFU/ml of sputum for Ultra versus 112.6 CFU/ml of sputum for Xpert, and for M. bovis BCG, it was 143.4 CFU/ml of sputum for Ultra versus 344 CFU/ml of sputum for Xpert. Ultra resulted in no false-positive RIF-R specimens, while Xpert resulted in two false-positive RIF-R specimens. All RIF-R-associated M. tuberculosis rpoB mutations tested were identified by Ultra. Testing on clinical sputum samples, Ultra versus Xpert, resulted in an overall sensitivity of 87.5% (95% confidence interval [CI], 82.1, 91.7) versus 81.0% (95% CI, 74.9, 86.2) and a sensitivity on sputum smear-negative samples of 78.9% (95% CI, 70.0, 86.1) versus 66.1% (95% CI, 56.4, 74.9). Both tests had a specificity of 98.7% (95% CI, 93.0, 100), and both had comparable accuracies for detection of RIF-R in these samples. Ultra should significantly improve TB detection, especially in patients with paucibacillary disease, and may provide more-reliable RIF-R detection.IMPORTANCE The Xpert MTB/RIF assay (Xpert), the first point-of-care assay for tuberculosis (TB), was endorsed by the World Health Organization in December 2010. Since then, 23 million Xpert tests have been procured in 130 countries. Although Xpert showed high overall sensitivity and specificity with pulmonary samples, its sensitivity has been lower with smear-negative pulmonary samples and extrapulmonary samples. In addition, the prediction of rifampin resistance (RIF-R) in paucibacillary samples and for a few rpoB mutations has resulted in both false-positive and false-negative results. The present study is the first demonstration of the design features and operational characteristics of an improved Xpert Ultra assay. This study also shows that the Ultra format overcomes many of the known shortcomings of Xpert. The new assay should significantly improve TB detection, especially in patients with paucibacillary disease, and provide more-reliable detection of RIF-R.
Keywords: Xpert MTB/RIF Ultra; diagnosis; sensitive; tuberculosis.
Copyright © 2017 Chakravorty et al.
Figures
References
-
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229–237. doi: 10.1128/JCM.01463-09. - DOI - PMC - PubMed
-
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. - DOI - PMC - PubMed
-
- Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, Bekker LG, Wood R. 2011. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med 8:e1001067. doi: 10.1371/journal.pmed.1001067. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

