A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE
- PMID: 28851867
- PMCID: PMC5575165
- DOI: 10.1038/s41598-017-06907-4
A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE
Abstract
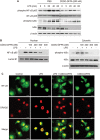
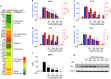
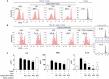
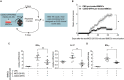
Tolerogenic dendritic cells (DCs) have emerged as relevant clinical targets for the treatment of multiple sclerosis and other autoimmune disorders. However, the pathways essential for conferring the tolerizing DC phenotype and optimal methods for their induction remain an intense area of research. Triterpenoids are a class of small molecules with potent immunomodulatory activity linked to activation of Nrf2 target genes, and can also suppress the manifestations of experimental autoimmune encephalomyelitis (EAE). Here we demonstrate that DCs are a principal target of the immune modulating activity of triterpenoids in the context of EAE. Exposure of DCs to the new class of triterpenoid CDDO-DFPA (RTA-408) results in the induction of HO-1, TGF-β, and IL-10, as well as the repression of NF-κB, EDN-1 and pro-inflammatory cytokines IL-6, IL-12, and TNFα. CDDO-DFPA exposed DCs retained expression of surface ligands and capacity for antigen uptake but were impaired to induce Th1 and Th17 cells. TGF-β was identified as the factor mediating suppression of T cell proliferation by CDDO-DFPA pretreated DCs, which failed to passively induce EAE. These findings demonstrate the potential therapeutic utility of CDDO-DFPA in the treatment and prevention of autoimmune disorders, and its capacity to induce tolerance via modulation of the DC phenotype.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

