VEGF amplifies transcription through ETS1 acetylation to enable angiogenesis
- PMID: 28851877
- PMCID: PMC5575285
- DOI: 10.1038/s41467-017-00405-x
VEGF amplifies transcription through ETS1 acetylation to enable angiogenesis
Abstract
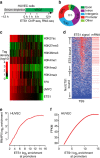
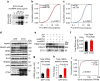
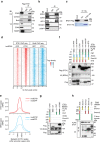
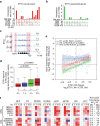
Release of promoter-proximally paused RNA polymerase II (RNAPII) is a recently recognized transcriptional regulatory checkpoint. The biological roles of RNAPII pause release and the mechanisms by which extracellular signals control it are incompletely understood. Here we show that VEGF stimulates RNAPII pause release by stimulating acetylation of ETS1, a master endothelial cell transcriptional regulator. In endothelial cells, ETS1 binds transcribed gene promoters and stimulates their expression by broadly increasing RNAPII pause release. 34 VEGF enhances ETS1 chromatin occupancy and increases ETS1 acetylation, enhancing its binding to BRD4, which recruits the pause release machinery and increases RNAPII pause release. Endothelial cell angiogenic responses in vitro and in vivo require ETS1-mediated transduction of VEGF signaling to release paused RNAPII. Our results define an angiogenic pathway in which VEGF enhances ETS1-BRD4 interaction to broadly promote RNAPII pause release and drive angiogenesis.Promoter proximal RNAPII pausing is a rate-limiting transcriptional mechanism. Chen et al. show that this process is essential in angiogenesis by demonstrating that the endothelial master transcription factor ETS1 promotes global RNAPII pause release, and that this process is governed by VEGF.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Activation of the VEGFR1 chromatin domain: an angiogenic signal-ETS1/HIF-2alpha regulatory axis.J Biol Chem. 2008 Sep 12;283(37):25404-25413. doi: 10.1074/jbc.M804349200. Epub 2008 Jul 14. J Biol Chem. 2008. PMID: 18625704 Free PMC article.
-
FGF9-induced ovarian cancer cell invasion involves VEGF-A/VEGFR2 augmentation by virtue of ETS1 upregulation and metabolic reprogramming.J Cell Biochem. 2018 Nov;119(10):8174-8189. doi: 10.1002/jcb.26820. Epub 2018 Jun 15. J Cell Biochem. 2018. PMID: 29904943
-
Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release.Mol Cell. 2021 May 20;81(10):2166-2182.e6. doi: 10.1016/j.molcel.2021.03.008. Epub 2021 Mar 24. Mol Cell. 2021. PMID: 33765415
-
Emerging roles of transcriptional enhancers in chromatin looping and promoter-proximal pausing of RNA polymerase II.J Biol Chem. 2018 Sep 7;293(36):13786-13794. doi: 10.1074/jbc.R117.813485. Epub 2017 Nov 29. J Biol Chem. 2018. PMID: 29187597 Free PMC article. Review.
-
The role of the transcription factor Ets1 in carcinoma.Semin Cancer Biol. 2015 Dec;35:20-38. doi: 10.1016/j.semcancer.2015.09.010. Epub 2015 Sep 25. Semin Cancer Biol. 2015. PMID: 26392377 Review.
Cited by
-
Identification of molecular markers for pre-thrombotic state: validation in the rabbits with tibia fracture or lung cancer.Transl Cancer Res. 2019 Oct;8(6):2316-2327. doi: 10.21037/tcr.2019.09.44. Transl Cancer Res. 2019. PMID: 35116984 Free PMC article.
-
Hypoxia-induced ELF3 promotes tumor angiogenesis through IGF1/IGF1R.EMBO Rep. 2022 Aug 3;23(8):e52977. doi: 10.15252/embr.202152977. Epub 2022 Jun 13. EMBO Rep. 2022. PMID: 35695065 Free PMC article.
-
Angiogenic Endothelial Cell Signaling in Cardiac Hypertrophy and Heart Failure.Front Cardiovasc Med. 2019 Mar 6;6:20. doi: 10.3389/fcvm.2019.00020. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 30895179 Free PMC article. Review.
-
Association of rs699947 (-2578 C/A) and rs2010963 (-634 G/C) Single Nucleotide Polymorphisms of the VEGF Gene, VEGF-A and Leptin Serum Level, and Cardiovascular Risk in Patients with Excess Body Mass: A Case-Control Study.J Clin Med. 2020 Feb 8;9(2):469. doi: 10.3390/jcm9020469. J Clin Med. 2020. PMID: 32046348 Free PMC article.
-
PARP inhibition promotes endothelial-like traits in melanoma cells and modulates pericyte coverage dynamics during vasculogenic mimicry.J Pathol. 2023 Mar;259(3):318-330. doi: 10.1002/path.6043. Epub 2023 Jan 4. J Pathol. 2023. PMID: 36484652 Free PMC article.
References
-
- Liu D, Jia H, Holmes DI, Stannard A, Zachary I. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler. Thromb. Vasc. Biol. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

