Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections
- PMID: 28851894
- PMCID: PMC5575141
- DOI: 10.1038/s41598-017-08311-4
Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections
Abstract
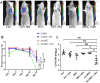
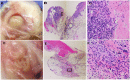
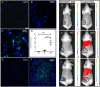
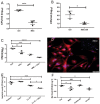
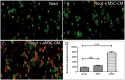
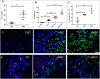
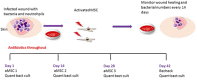
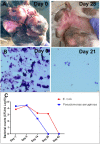
Chronic bacterial infections associated with biofilm formation are often difficult to resolve without extended courses of antibiotic therapy. Mesenchymal stem cells (MSC) exert antibacterial activity in vitro and in acute bacterial infection models, but their activity in chronic infection with biofilm models has not been previously investigated. Therefore, we studied the effects of MSC administration in mouse and dog models of chronic infections associated with biofilms. Mice with chronic Staphylococcus aureus implant infections were treated by i.v. administration of activated or non-activated MSC, with or without antibiotic therapy. The most effective treatment protocol was identified as activated MSC co-administered with antibiotic therapy. Activated MSC were found to accumulate in the wound margins several days after i.v. administration. Macrophages in infected tissues assumed an M2 phenotype, compared to untreated infections which contained predominately M1 macrophages. Bacterial killing by MSC was found to be mediated in part by secretion of cathelicidin and was significantly increased by antibiotics. Studies in pet dogs with spontaneous chronic multi drug-resistant wound infections demonstrated clearance of bacteria and wound healing following repeated i.v. administration of activated allogeneic canine MSC. Thus, systemic therapy with activated MSC may be an effective new, non-antimicrobial approach to treatment of chronic, drug-resistant infections.
Conflict of interest statement
Two authors on the manuscript (V.J. and S.D.) are named as co-inventors on a patent that has been filed covering the antimicrobial stem cell technology.
Figures
References
-
- Garwood CS, Steinberg JS. What’s new in wound treatment: a critical appraisal. Diabetes Metab Res Rev. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

