Prion protein facilitates retinal iron uptake and is cleaved at the β-site: Implications for retinal iron homeostasis in prion disorders
- PMID: 28851903
- PMCID: PMC5575325
- DOI: 10.1038/s41598-017-08821-1
Prion protein facilitates retinal iron uptake and is cleaved at the β-site: Implications for retinal iron homeostasis in prion disorders
Abstract
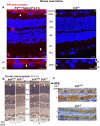
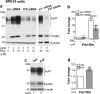
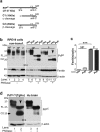
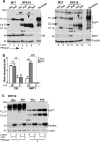
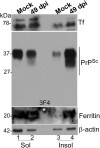
Prion disease-associated retinal degeneration is attributed to PrP-scrapie (PrPSc), a misfolded isoform of prion protein (PrPC) that accumulates in the neuroretina. However, a lack of temporal and spatial correlation between PrPSc and cytotoxicity suggests the contribution of host factors. We report retinal iron dyshomeostasis as one such factor. PrPC is expressed on the basolateral membrane of retinal-pigment-epithelial (RPE) cells, where it mediates uptake of iron by the neuroretina. Accordingly, the neuroretina of PrP-knock-out mice is iron-deficient. In RPE19 cells, silencing of PrPC decreases ferritin while over-expression upregulates ferritin and divalent-metal-transporter-1 (DMT-1), indicating PrPC-mediated iron uptake through DMT-1. Polarization of RPE19 cells results in upregulation of ferritin by ~10-fold and β-cleavage of PrPC, the latter likely to block further uptake of iron due to cleavage of the ferrireductase domain. A similar β-cleavage of PrPC is observed in mouse retinal lysates. Scrapie infection causes PrPSc accumulation and microglial activation, and surprisingly, upregulation of transferrin despite increased levels of ferritin. Notably, detergent-insoluble ferritin accumulates in RPE cells and correlates temporally with microglial activation, not PrPSc accumulation, suggesting that impaired uptake of iron by PrPSc combined with inflammation results in retinal iron-dyshomeostasis, a potentially toxic host response contributing to prion disease-associated pathology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Prion protein modulates glucose homeostasis by altering intracellular iron.Sci Rep. 2018 Apr 26;8(1):6556. doi: 10.1038/s41598-018-24786-1. Sci Rep. 2018. PMID: 29700330 Free PMC article.
-
Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson's disease.Free Radic Biol Med. 2016 Aug;97:292-306. doi: 10.1016/j.freeradbiomed.2016.06.025. Epub 2016 Jun 23. Free Radic Biol Med. 2016. PMID: 27343690 Free PMC article.
-
The Role of Prion Protein in Reelin/Dab1 Signaling: Implications for Neurodegeneration.Viruses. 2025 Jun 29;17(7):928. doi: 10.3390/v17070928. Viruses. 2025. PMID: 40733546 Free PMC article.
-
Prions and prion diseases: Insights from the eye.Exp Eye Res. 2020 Oct;199:108200. doi: 10.1016/j.exer.2020.108200. Epub 2020 Aug 25. Exp Eye Res. 2020. PMID: 32858007 Free PMC article. Review.
-
[Systematic review of the therapeutics for prion diseases].Brain Nerve. 2009 Aug;61(8):929-38. Brain Nerve. 2009. PMID: 19697882 Japanese.
Cited by
-
Beta-endoproteolysis of the cellular prion protein by dipeptidyl peptidase-4 and fibroblast activation protein.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2209815120. doi: 10.1073/pnas.2209815120. Epub 2022 Dec 27. Proc Natl Acad Sci U S A. 2023. PMID: 36574660 Free PMC article.
-
Prion protein modulates iron transport in the anterior segment: Implications for ocular iron homeostasis and prion transmission.Exp Eye Res. 2018 Oct;175:1-13. doi: 10.1016/j.exer.2018.05.031. Epub 2018 May 31. Exp Eye Res. 2018. PMID: 29859760 Free PMC article.
-
Prion Protein Endoproteolysis: Cleavage Sites, Mechanisms and Connections to Prion Disease.J Neurochem. 2025 Jan;169(1):e16310. doi: 10.1111/jnc.16310. J Neurochem. 2025. PMID: 39874431 Free PMC article. Review.
-
β-Cleavage of the prion protein in the human eye: Implications for the spread of infectious prions and human ocular disorders.Exp Eye Res. 2021 Nov;212:108787. doi: 10.1016/j.exer.2021.108787. Epub 2021 Oct 7. Exp Eye Res. 2021. PMID: 34624335 Free PMC article.
-
Prion protein modulates glucose homeostasis by altering intracellular iron.Sci Rep. 2018 Apr 26;8(1):6556. doi: 10.1038/s41598-018-24786-1. Sci Rep. 2018. PMID: 29700330 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

