Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization
- PMID: 28851929
- PMCID: PMC5574979
- DOI: 10.1038/s41598-017-09260-8
Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization
Abstract
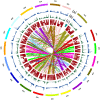
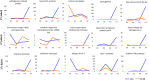
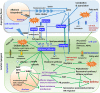
Aflatoxin contamination, caused by fungal pathogen Aspergillus flavus, is a major quality and health problem delimiting the trade and consumption of groundnut (Arachis hypogaea L.) worldwide. RNA-seq approach was deployed to understand the host-pathogen interaction by identifying differentially expressed genes (DEGs) for resistance to in-vitro seed colonization (IVSC) at four critical stages after inoculation in J 11 (resistant) and JL 24 (susceptible) genotypes of groundnut. About 1,344.04 million sequencing reads have been generated from sixteen libraries representing four stages in control and infected conditions. About 64% and 67% of quality filtered reads (1,148.09 million) were mapped onto A (A. duranensis) and B (A. ipaёnsis) subgenomes of groundnut respectively. About 101 million unaligned reads each from J 11 and JL 24 were used to map onto A. flavus genome. As a result, 4,445 DEGs including defense-related genes like senescence-associated proteins, resveratrol synthase, 9s-lipoxygenase, pathogenesis-related proteins were identified. In A. flavus, about 578 DEGs coding for growth and development of fungus, aflatoxin biosynthesis, binding, transport, and signaling were identified in compatible interaction. Besides identifying candidate genes for IVSC resistance in groundnut, the study identified the genes involved in host-pathogen cross-talks and markers that can be used in breeding resistant varieties.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Andrade P, Caldas E. Aflatoxins in cereals: worldwide occurrence and dietary risk assessment. World Mycotoxin Journal. 2015;8:415–431. doi: 10.3920/WMJ2014.1847. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

