A Plasmodium plasma membrane reporter reveals membrane dynamics by live-cell microscopy
- PMID: 28851956
- PMCID: PMC5575152
- DOI: 10.1038/s41598-017-09569-4
A Plasmodium plasma membrane reporter reveals membrane dynamics by live-cell microscopy
Abstract
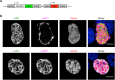
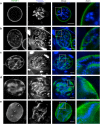
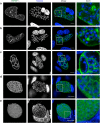
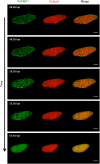
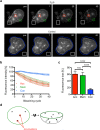
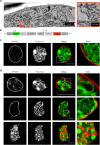
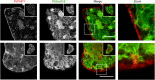
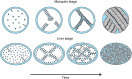
During asexual replication within the Anopheles mosquito and their vertebrate host, Plasmodium parasites depend on the generation of a massive amount of new plasma membrane to produce thousands of daughter parasites. How the parasite plasma membrane (PPM) is formed has mostly been studied by electron microscopy, which does not allow an insight into the dynamics of this process. We generated a Plasmodium berghei reporter parasite line by GFP-tagging of a non-essential PPM-localized protein, and followed plasma membrane development in living parasites through the entire Plasmodium life cycle. By generating double-fluorescent parasites in which the PPM is visualized in combination with the parasite endoplasmic reticulum, we show that membrane contact sites are formed between both membrane systems during oocyst and liver stage development that might be used to deliver lipids to the dramatically expanding PPM. In conclusion, we have established a powerful tool to follow PPM development in living parasites, which promises to greatly expand our knowledge of membrane biology in the Plasmodium parasite.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Organelle segregation into Plasmodium liver stage merozoites.Cell Microbiol. 2011 Nov;13(11):1768-82. doi: 10.1111/j.1462-5822.2011.01657.x. Epub 2011 Aug 31. Cell Microbiol. 2011. PMID: 21801293
-
A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle.Mol Biochem Parasitol. 2004 Sep;137(1):23-33. doi: 10.1016/j.molbiopara.2004.04.007. Mol Biochem Parasitol. 2004. PMID: 15279948
-
GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites.Biol Cell. 2009 Jul;101(7):415-30, 5 p following 430. doi: 10.1042/BC20080202. Biol Cell. 2009. PMID: 19143588
-
The complex interplay between mosquito positive and negative regulators of Plasmodium development.Curr Opin Microbiol. 2005 Aug;8(4):415-21. doi: 10.1016/j.mib.2005.06.013. Curr Opin Microbiol. 2005. PMID: 15996894 Review.
-
Let it glow: genetically encoded fluorescent reporters in Plasmodium.Malar J. 2024 Apr 20;23(1):114. doi: 10.1186/s12936-024-04936-9. Malar J. 2024. PMID: 38643106 Free PMC article. Review.
Cited by
-
Asynchronous nuclear cycles in multinucleated Plasmodium falciparum facilitate rapid proliferation.Sci Adv. 2022 Apr;8(13):eabj5362. doi: 10.1126/sciadv.abj5362. Epub 2022 Mar 30. Sci Adv. 2022. PMID: 35353560 Free PMC article.
-
A Plasmodium homolog of ER tubule-forming proteins is required for parasite virulence.Mol Microbiol. 2020 Sep;114(3):454-467. doi: 10.1111/mmi.14526. Epub 2020 Jun 19. Mol Microbiol. 2020. PMID: 32432369 Free PMC article.
-
The Plasmodium circumsporozoite protein.Trends Parasitol. 2024 Dec;40(12):1124-1134. doi: 10.1016/j.pt.2024.10.017. Epub 2024 Nov 20. Trends Parasitol. 2024. PMID: 39572325 Free PMC article. Review.
-
BioID Reveals Novel Proteins of the Plasmodium Parasitophorous Vacuole Membrane.mSphere. 2018 Jan 24;3(1):e00522-17. doi: 10.1128/mSphere.00522-17. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29404413 Free PMC article.
-
Plasmodium schizogony, a chronology of the parasite's cell cycle in the blood stage.PLoS Pathog. 2023 Mar 2;19(3):e1011157. doi: 10.1371/journal.ppat.1011157. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36862652 Free PMC article. Review.
References
-
- World Health Organization. World Malaria Report (2016).
-
- De Niz, M. et al. Progress in imaging methods: insights gained into Plasmodium biology. Nat. Rev. Microbiol. 15, 37–54. (2016). - PubMed
-
- Ward GE, Miller LH, Dvorak JA. The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci. 1993;106:237–248. - PubMed
-
- Stanway, R. R., Witt, T., Zobiak, B., Aepfelbacher, M. & Heussler, V. T. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol. Cell101, 415–430, 5 p following 430 (2009). - PubMed
-
- Stanway, R. R. et al. Organelle segregation into Plasmodium liver stage merozoites. Cell. Microbiol.13, 1768–1782 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

