Linoleic acid metabolite leads to steroid resistant asthma features partially through NF-κB
- PMID: 28851976
- PMCID: PMC5575291
- DOI: 10.1038/s41598-017-09869-9
Linoleic acid metabolite leads to steroid resistant asthma features partially through NF-κB
Abstract
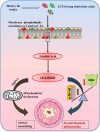
Studies have highlighted the role of nutritional and metabolic modulators in asthma pathobiology. Steroid resistance is an important clinical problem in asthma but lacks good experimental models. Linoleic acid, a polyunsaturated fatty acid, has been linked to asthma and glucocorticoid sensitivity. Its 12/15-lipoxygenase metabolite, 13-S-hydroxyoctadecadienoic acid (HODE) induces mitochondrial dysfunction, with severe airway obstruction and neutrophilic airway inflammation. Here we show that HODE administration leads to steroid unresponsiveness in an otherwise steroid responsive model of allergic airway inflammation (AAI). HODE treatment to allergic mice further increased airway hyperresponsiveness and goblet metaplasia. Treatment with dexamethasone was associated with increased neutrophilic inflammation in HODE treated allergic mice; unlike control allergic mice that showed resolution of inflammation. HODE induced loss of steroid sensitivity was associated with increased p-NFkB in mice and reduced GR-α transcript levels in cultured human bronchial epithelia. In summary, HODE modifies typical AAI to recapitulate many of the phenotypic features seen in severe steroid unresponsive asthma. We speculate that since HODE is a natural metabolite, it may be relevant to the increased asthma severity and steroid insensitivity in patients who are obese or consume high fat diets. Further characterization of HODE induced steroid insensitivity may clarify the mechanisms.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
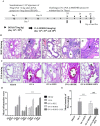
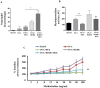
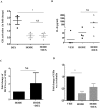
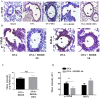


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

