Hypoxic Stress Decreases c-Myc Protein Stability in Cardiac Progenitor Cells Inducing Quiescence and Compromising Their Proliferative and Vasculogenic Potential
- PMID: 28851980
- PMCID: PMC5575078
- DOI: 10.1038/s41598-017-09813-x
Hypoxic Stress Decreases c-Myc Protein Stability in Cardiac Progenitor Cells Inducing Quiescence and Compromising Their Proliferative and Vasculogenic Potential
Abstract
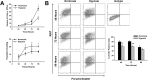
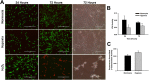
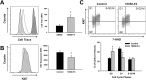
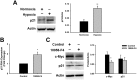
Cardiac progenitor cells (CPCs) have been shown to promote cardiac regeneration and improve heart function. However, evidence suggests that their regenerative capacity may be limited in conditions of severe hypoxia. Elucidating the mechanisms involved in CPC protection against hypoxic stress is essential to maximize their cardioprotective and therapeutic potential. We investigated the effects of hypoxic stress on CPCs and found significant reduction in proliferation and impairment of vasculogenesis, which were associated with induction of quiescence, as indicated by accumulation of cells in the G0-phase of the cell cycle and growth recovery when cells were returned to normoxia. Induction of quiescence was associated with a decrease in the expression of c-Myc through mechanisms involving protein degradation and upregulation of p21. Inhibition of c-Myc mimicked the effects of severe hypoxia on CPC proliferation, also triggering quiescence. Surprisingly, these effects did not involve changes in p21 expression, indicating that other hypoxia-activated factors may induce p21 in CPCs. Our results suggest that hypoxic stress compromises CPC function by inducing quiescence in part through downregulation of c-Myc. In addition, we found that c-Myc is required to preserve CPC growth, suggesting that modulation of pathways downstream of it may re-activate CPC regenerative potential under ischemic conditions.
Conflict of interest statement
Dr. Hare has a patent for cardiac cell-based therapy; he holds equity in Vestion Inc.; maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board; and is a shareholder in Longeveron LLC. The other authors declare that they have no competing interests.
Figures



Similar articles
-
Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction.Stem Cells Transl Med. 2016 Aug;5(8):1067-78. doi: 10.5966/sctm.2015-0281. Epub 2016 Jun 22. Stem Cells Transl Med. 2016. PMID: 27334489 Free PMC article.
-
Decline in cellular function of aged mouse c-kit+ cardiac progenitor cells.J Physiol. 2017 Oct 1;595(19):6249-6262. doi: 10.1113/JP274775. Epub 2017 Aug 18. J Physiol. 2017. PMID: 28737214 Free PMC article.
-
Changes of Metabolic Phenotype of Cardiac Progenitor Cells During Differentiation: Neutral Effect of Stimulation of AMP-Activated Protein Kinase.Stem Cells Dev. 2019 Nov 15;28(22):1498-1513. doi: 10.1089/scd.2019.0129. Epub 2019 Oct 22. Stem Cells Dev. 2019. PMID: 31530214
-
Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation.Cell Death Differ. 2008 Apr;15(4):672-7. doi: 10.1038/sj.cdd.4402302. Epub 2008 Jan 11. Cell Death Differ. 2008. PMID: 18188166 Review.
-
The mysterious human epidermal cell cycle, or an oncogene-induced differentiation checkpoint.Cell Cycle. 2012 Dec 15;11(24):4507-16. doi: 10.4161/cc.22529. Epub 2012 Oct 31. Cell Cycle. 2012. PMID: 23114621 Free PMC article. Review.
Cited by
-
Fetal Hypoxia Impacts on Proliferation and Differentiation of Sca-1+ Cardiac Progenitor Cells and Maturation of Cardiomyocytes: A Role of MicroRNA-210.Genes (Basel). 2020 Mar 20;11(3):328. doi: 10.3390/genes11030328. Genes (Basel). 2020. PMID: 32244901 Free PMC article.
-
MYC: there is more to it than cancer.Front Cell Dev Biol. 2024 Mar 6;12:1342872. doi: 10.3389/fcell.2024.1342872. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38510176 Free PMC article. Review.
-
Metabolic Roles of HIF1, c-Myc, and p53 in Glioma Cells.Metabolites. 2024 Apr 25;14(5):249. doi: 10.3390/metabo14050249. Metabolites. 2024. PMID: 38786726 Free PMC article. Review.
References
-
- Lloyd-Jones, D. et al. and the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics 2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation119, e21–e181 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

