Tcf7l2 plays pleiotropic roles in the control of glucose homeostasis, pancreas morphology, vascularization and regeneration
- PMID: 28851992
- PMCID: PMC5575064
- DOI: 10.1038/s41598-017-09867-x
Tcf7l2 plays pleiotropic roles in the control of glucose homeostasis, pancreas morphology, vascularization and regeneration
Abstract
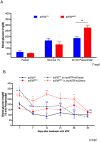
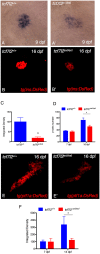
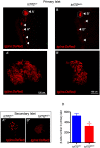
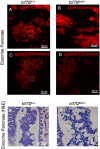
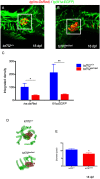
Type 2 diabetes (T2D) is a disease characterized by impaired insulin secretion. The Wnt signaling transcription factor Tcf7l2 is to date the T2D-associated gene with the largest effect on disease susceptibility. However, the mechanisms by which TCF7L2 variants affect insulin release from β-cells are not yet fully understood. By taking advantage of a tcf7l2 zebrafish mutant line, we first show that these animals are characterized by hyperglycemia and impaired islet development. Moreover, we demonstrate that the zebrafish tcf7l2 gene is highly expressed in the exocrine pancreas, suggesting potential bystander effects on β-cell growth, differentiation and regeneration. Finally, we describe a peculiar vascular phenotype in tcf7l2 mutant larvae, characterized by significant reduction in the average number and diameter of pancreatic islet capillaries. Overall, the zebrafish Tcf7l2 mutant, characterized by hyperglycemia, pancreatic and vascular defects, and reduced regeneration proves to be a suitable model to study the mechanism of action and the pleiotropic effects of Tcf7l2, the most relevant T2D GWAS hit in human populations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

