Evolutionary conserved role of eukaryotic translation factor eIF5A in the regulation of actin-nucleating formins
- PMID: 28852021
- PMCID: PMC5575014
- DOI: 10.1038/s41598-017-10057-y
Evolutionary conserved role of eukaryotic translation factor eIF5A in the regulation of actin-nucleating formins
Abstract
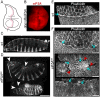
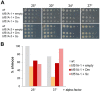
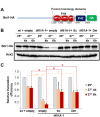
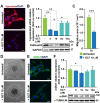
Elongation factor eIF5A is required for the translation of consecutive prolines, and was shown in yeast to translate polyproline-containing Bni1, an actin-nucleating formin required for polarized growth during mating. Here we show that Drosophila eIF5A can functionally replace yeast eIF5A and is required for actin-rich cable assembly during embryonic dorsal closure (DC). Furthermore, Diaphanous, the formin involved in actin dynamics during DC, is regulated by and mediates eIF5A effects. Finally, eIF5A controls cell migration and regulates Diaphanous levels also in mammalian cells. Our results uncover an evolutionary conserved role of eIF5A regulating cytoskeleton-dependent processes through translation of formins in eukaryotes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Role of eIF5A in Mitochondrial Function.Int J Mol Sci. 2022 Jan 24;23(3):1284. doi: 10.3390/ijms23031284. Int J Mol Sci. 2022. PMID: 35163207 Free PMC article. Review.
-
Fertility and polarized cell growth depends on eIF5A for translation of polyproline-rich formins in Saccharomyces cerevisiae.Genetics. 2014 Aug;197(4):1191-200. doi: 10.1534/genetics.114.166926. Epub 2014 Jun 11. Genetics. 2014. PMID: 24923804 Free PMC article.
-
Deoxyhypusine Modification of Eukaryotic Translation Initiation Factor 5A (eIF5A) Is Essential for Trypanosoma brucei Growth and for Expression of Polyprolyl-containing Proteins.J Biol Chem. 2015 Aug 7;290(32):19987-98. doi: 10.1074/jbc.M115.656785. Epub 2015 Jun 16. J Biol Chem. 2015. PMID: 26082486 Free PMC article.
-
The polyproline-motif of S6K2: eIF5A translational dependence and importance for protein-protein interactions.J Cell Biochem. 2019 Apr;120(4):6015-6025. doi: 10.1002/jcb.27888. Epub 2018 Oct 15. J Cell Biochem. 2019. PMID: 30320934
-
Insights into eukaryotic translation initiation factor 5A: Its role and mechanisms in protein synthesis.Biochim Biophys Acta Mol Cell Res. 2024 Dec;1871(8):119849. doi: 10.1016/j.bbamcr.2024.119849. Epub 2024 Sep 19. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 39303786 Review.
Cited by
-
Sequencing the mosaic genome of Brahman cattle identifies historic and recent introgression including polled.Sci Rep. 2018 Dec 10;8(1):17761. doi: 10.1038/s41598-018-35698-5. Sci Rep. 2018. PMID: 30531891 Free PMC article.
-
Role of eIF5A in Mitochondrial Function.Int J Mol Sci. 2022 Jan 24;23(3):1284. doi: 10.3390/ijms23031284. Int J Mol Sci. 2022. PMID: 35163207 Free PMC article. Review.
-
Eukaryotic Initiation Factor 5A2 localizes to actively translating ribosomes to promote cancer cell protrusions and invasive capacity.Cell Commun Signal. 2023 Mar 13;21(1):54. doi: 10.1186/s12964-023-01076-6. Cell Commun Signal. 2023. PMID: 36915194 Free PMC article.
-
SUMOylation modulates eIF5A activities in both yeast and pancreatic ductal adenocarcinoma cells.Cell Mol Biol Lett. 2024 Jan 16;29(1):15. doi: 10.1186/s11658-024-00533-5. Cell Mol Biol Lett. 2024. PMID: 38229033 Free PMC article.
-
Yeast Translation Elongation Factor eIF5A Expression Is Regulated by Nutrient Availability through Different Signalling Pathways.Int J Mol Sci. 2020 Dec 28;22(1):219. doi: 10.3390/ijms22010219. Int J Mol Sci. 2020. PMID: 33379337 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

