Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse
- PMID: 28852050
- PMCID: PMC5575033
- DOI: 10.1038/s41598-017-10213-4
Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse
Abstract
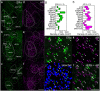
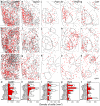
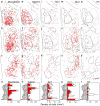
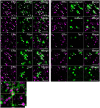
Testosterone is involved in male sexual, parental and aggressive behaviors through the androgen receptor (AR) and estrogen receptor (ER) α expressed in the brain. Although several studies have demonstrated that ERα and AR in the medial preoptic area (MPOA) are required for exhibiting sexual and aggressive behaviors of male mice, the molecular characteristics of ERα- and AR-expressing cells in the mouse MPOA are largely unknown. Here, we performed in situ hybridization for neurotransmitters and neuropeptides, combined with immunohistochemistry for ERα and AR to quantitate and characterize gonadal steroid receptor-expressing cells in the MPOA subregions of male mice. Prodynorphin, preproenkephalin (Penk), cocaine- and amphetamine-related transcript, neurotensin, galanin, tachykinin (Tac)1, Tac2 and thyrotropin releasing hormone (Trh) have distinct expression patterns in the MPOA subregions. Gad67-expressing cells were the most dominant neuronal subtype among the ERα- and AR-expressing cells throughout the MPOA. The percentage of ERα- and AR-immunoreactivities varied depending on the neuronal subtype. A substantial proportion of the neurotensin-, galanin-, Tac2- and Penk-expressing cells in the MPOA were positive for ERα and AR, whereas the vast majority of the Trh-expressing cells were negative. These results suggest that testosterone exerts differential effects depending on both the neuronal subtypes and MPOA subregions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

