Phosphatidylinositol-3 Kinase Inhibitors, Buparlisib and Alpelisib, Sensitize Estrogen Receptor-positive Breast Cancer Cells to Tamoxifen
- PMID: 28852212
- PMCID: PMC5574981
- DOI: 10.1038/s41598-017-10555-z
Phosphatidylinositol-3 Kinase Inhibitors, Buparlisib and Alpelisib, Sensitize Estrogen Receptor-positive Breast Cancer Cells to Tamoxifen
Abstract
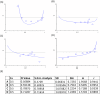
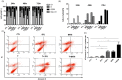
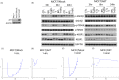
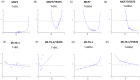
Tamoxifen is the standard first-line hormonal therapy for premenopausal women with estrogen receptor (ER)-positive metastatic breast cancer (BC). One of the crucial mechanisms underlying hormonal therapy resistance is the collateral activation of the phosphatidylinositol-3 kinase (PI3K)/AKT pathway. We explored whether PI3K inhibitors, buparlisib and alpelisib, enhance the efficacy of tamoxifen against ER-positive BC cells. We have observed a synergism between alpelisib or buparlisib and tamoxifen in the treatment for ER-positive BC cell lines harboring different PI3K alterations. Immunoblotting analysis showed alpelisib, buparlisib, or either drug in combination with tamoxifen downregulated the PI3K downstream targets in the MCF-7 and ZR75-1 cells. In the MCF-7 cells transfected with a constitutive active (myristoylated) AKT1 construct or mutant ER, the synergistic effect between alpelisib and tamoxifen was markedly attenuated, indicating that synergism depends on AKT inhibition or normally functioning ER. Combining alpelisib or buparlisib with tamoxifen also attenuated MCF-7 tumor growth in Balb/c nude mice. Our data suggest that additional PI3K blockade might be effective in enhancing the therapeutic effect of tamoxifen in ER-positive BC and support the rationale combination in clinical trials.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

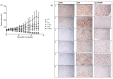
References
-
- Kaufmann M, et al. Goserelin, a depot gonadotrophin-releasing hormone agonist in the treatment of premenopausal patients with metastatic breast cancer. German Zoladex Trial Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1989;7:1113–1119. doi: 10.1200/JCO.1989.7.8.1113. - DOI - PubMed
-
- Klijn JG, et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:343–353. doi: 10.1200/JCO.2001.19.2.343. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

