Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study
- PMID: 28852318
- PMCID: PMC5558122
- DOI: 10.3748/wjg.v23.i30.5589
Efficacy and safety of Xiangsha Liujunzi granules for functional dyspepsia: A multi-center randomized double-blind placebo-controlled clinical study
Abstract
Aim: To assess the efficacy and safety of a Chinese herbal medicine (CHM), Xiangsha Liujunzi granules, in the treatment of patients with functional dyspepsia (FD).
Methods: We performed a randomized, double-blind, placebo-controlled trial with patients from three centers. Two hundred and sixteen subjects diagnosed with FD according to ROME III criteria and confirmed by upper gastrointestinal endoscopy and spleen-deficiency and Qi-stagnation syndrome were selected to receive Xiangsha Liujunzi granules or placebo for 4 wk in a 2:1 ratio by blocked randomization. The subjects also received follow-up after the 4-wk intervention. Herbal or placebo granules were dissolved in 300 mL of water. Participants in both groups were administered 130 mL (45 °C) three times a day. Participants were evaluated prior to and following 4 wk of the intervention in terms of changes in the postprandial discomfort severity scale (PDSS) score, clinical global impression (CGI) scale score, hospital anxiety and depression scale (HADS) score, traditional Chinese medicine symptoms score (SS), scores of various domains of the 36-item short form health survey (SF-36), gastric emptying (GE) and any observed adverse effects.
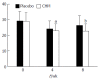
Results: Compared with the placebo group, patients in the CHM group showed significant improvements in the scores of PDSS, HADS, SS, SF-36 and CGI scale (P < 0.05 or P < 0.01). They also showed the amelioration in the GE rates of the proximal stomach and distal stomach (P < 0.05 or P < 0.01).
Conclusion: Xiangsha Liujunzi granules offered significant symptomatic improvement in patients with FD.
Keywords: Chinese herbal medicine; Efficacy; Functional dyspepsia; Randomized controlled trial; Xiangsha Liujunzi.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no competing interests. Neither the funding agency nor any outside organization has a role in study design or manuscript preparation.
Figures

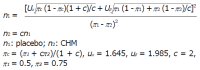






References
-
- Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:187–194. - PubMed
-
- Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10:134–141. - PubMed
-
- Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, Harmsen WS, Zinsmeister AR, Agréus L. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175–1183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

