Influence of 45S5 Bioactive Glass in A Standard Calcium Phosphate Collagen Bone Graft Substitute on the Posterolateral Fusion of Rabbit Spine
- PMID: 28852357
- PMCID: PMC5508293
Influence of 45S5 Bioactive Glass in A Standard Calcium Phosphate Collagen Bone Graft Substitute on the Posterolateral Fusion of Rabbit Spine
Abstract
Introduction: Spinal fusion surgery is an effective but costly treatment for select spinal pathology. Historically iliac crest bone graft (ICBG) has remained the gold standard for achieving successful arthrodesis. Given well-established morbidity autograft harvest, multiple bone graft replacements, void fillers, and extenders have been developed. The objective of this study was to evaluate the in vivo efficacy and safety of two mineralized collagen bone void filler materials similar in composition. Both bone void fillers were composed of hydroxyapatite (HA), tricalcium phosphate (TCP) and bovine collagen. The first test article (Bi-Ostetic bioactive glass foam or "45S5") also contained 45S5 bioactive glass particles while the second test article (Formagraft or "FG") did not. 45S5 and FG were combined with bone marrow aspirate and iliac crest autograft and compared to ICBG in an established posterolateral spine fusion rabbit model.
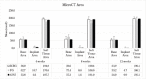
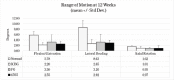
Materials and methods: Sixty-nine mature New Zealand White rabbits were divided into 3 test cohorts: ICBG, 45S5, and FG. A Posterolateral fusion model previous validated was utilized to assess fusion efficacy. The test groups were evaluated for spine fusion rate, new bone formation, graft resorption and inflammatory response using radiographic, μCT, biomechanical and histological endpoints at 4, 8 and 12 weeks following implantation.
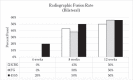
Results: There were 4 clinical complications unrelated to the graft materials and were evenly split between groups (ICBG graft harvest complications; hind limb mobility, chronic pain) and were euthanized. These omissions did not affect the overall outcome of the study. Radiographic scoring of the fusion sites indicated a normal healing response in all test groups, with no adverse reactions and similar progressions of new bone formation observed over time. All groups demonstrated significantly less range of motion in both flexion/extension and lateral bending compared to normal not-fused controls, which supports fusion results observed in the other endpoints. Fusion occurred earlier in the 45S5 group: ICBG 0%, FG 0%, and 45S5 20% at 4 weeks; ICBG 43%, FG 38%, and 45S5 50% at 8 weeks; and ICBG 50%, FG 56%, and 45S5 56% at 12 weeks. Histopathology analysis of the fusion masses, from each test article and time point, indicated an expected normal response for resorbable calcium phosphate (HA/TCP) and collagen graft material. Mild inflammation with macrophage and multinucleated giant cell response to the graft material was evident in all test groups.
Discussion: This study has confirmed the biocompatibility, safety, efficacy and bone healing characteristics of the HA-TCP collagen (with or without 45S5 bioactive glass) composites. The results show that the 3 test groups had equivalent long-term fusion performance and outcome at 12 weeks. However, the presence of 45S5 bioactive glass seemed to accelerate the fusion process as evidenced by the higher fusion rates at 4 and 8 weeks for the HA-TCP-collagen composite containing bioactive glass particles. The results also demonstrate that the HA-TCP-45S5 bioactive glass-collagen composite used as an extender closely mirrors the healing characteristics (i.e. amount and quality of bone) of the 100% autograft group.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures

Similar articles
-
Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model.Iowa Orthop J. 2019;39(2):9-19. Iowa Orthop J. 2019. PMID: 32577102 Free PMC article.
-
Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model.Iowa Orthop J. 2016;36:167-73. Iowa Orthop J. 2016. PMID: 27528855 Free PMC article.
-
Radiographic, biomechanical, and histological evaluation of rhBMP-2 in a 3-level intertransverse process spine fusion: an ovine study.J Neurosurg Spine. 2016 Dec;25(6):733-739. doi: 10.3171/2016.4.SPINE151316. Epub 2016 Jul 1. J Neurosurg Spine. 2016. PMID: 27367941
-
Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion.J Neurosurg Spine. 2014 Jul;21(1):106-32. doi: 10.3171/2014.4.SPINE14325. J Neurosurg Spine. 2014. PMID: 24980593 Review.
-
Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel.Spine (Phila Pa 1976). 1998 Jan 15;23(2):159-67. doi: 10.1097/00007632-199801150-00003. Spine (Phila Pa 1976). 1998. PMID: 9474720 Review.
Cited by
-
Bioactive Glass Graft vs Allograft in Benign Bone Lesions: A Retrospective Comparative Study.HSS J. 2025 Mar 20:15563316251321825. doi: 10.1177/15563316251321825. Online ahead of print. HSS J. 2025. PMID: 40125295 Free PMC article.
-
Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model.Iowa Orthop J. 2019;39(2):9-19. Iowa Orthop J. 2019. PMID: 32577102 Free PMC article.
-
The Biocompatibility of a New Type of 45S5 Bioactive Graft in a Sheep Model: A Pilot Study.Cureus. 2023 Jul 7;15(7):e41521. doi: 10.7759/cureus.41521. eCollection 2023 Jul. Cureus. 2023. PMID: 37551216 Free PMC article.
-
Use of tendon to produce decellularized sheets of mineralized collagen fibrils for bone tissue repair and regeneration.J Biomed Mater Res B Appl Biomater. 2020 Apr;108(3):845-856. doi: 10.1002/jbm.b.34438. Epub 2019 Jun 26. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31241254 Free PMC article.
-
Efficacy of a synthetic calcium phosphate with submicron surface topography as autograft extender in lapine posterolateral spinal fusion.J Biomed Mater Res B Appl Biomater. 2019 Aug;107(6):2080-2090. doi: 10.1002/jbm.b.34301. Epub 2019 Jan 7. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30614621 Free PMC article.
References
-
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–9. - PubMed
-
- Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9(3):210–8. - PubMed
-
- Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res. 1994;309:208–13. - PubMed
-
- Kahn B. Superior gluteal artery laceration, a complication of iliac bone graft surgery. Clin Orthop Relat Res. 1979;140:204–7. - PubMed
-
- Kurz LT, Garfin SR, Booth RE., Jr. Harvesting autogenous iliac bone grafts. A review of complications and techniques Spine (Phila Pa 1976) 1989;14(12):1324–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
